Lecture 13 - De Novo Molecule Generation#
Learning goals#
Connect unsupervised learning ideas to molecular generation.
Explain what an encoder and a decoder are.
Understand the Variational Autoencoder (VAE) idea and why it helps sampling.
Train a small VAE on SMILES and generate new molecules.
Inspect what encode outputs look like and how sampling works in latent space.
For this lecture 10, it is recommended to run everything in Colab.
1. Setup and data#
Similar to Lecture 11, we will first build a 10 descriptor dataset for our 575 molecules loaded from C-H oxidation dataset.
url = "https://raw.githubusercontent.com/zzhenglab/ai4chem/main/book/_data/C_H_oxidation_dataset.csv"
df_raw = pd.read_csv(url)
df_raw.head(3)
def calc_descriptors10(smiles: str):
m = Chem.MolFromSmiles(smiles)
return pd.Series({
"MolWt": Descriptors.MolWt(m),
"LogP": Crippen.MolLogP(m),
"TPSA": rdMolDescriptors.CalcTPSA(m),
"NumRings": rdMolDescriptors.CalcNumRings(m),
"NumHAcceptors": rdMolDescriptors.CalcNumHBA(m),
"NumHDonors": rdMolDescriptors.CalcNumHBD(m),
"NumRotatableBonds": rdMolDescriptors.CalcNumRotatableBonds(m),
"HeavyAtomCount": Descriptors.HeavyAtomCount(m),
"FractionCSP3": rdMolDescriptors.CalcFractionCSP3(m),
"NumAromaticRings": rdMolDescriptors.CalcNumAromaticRings(m)
})
desc10 = df_raw["SMILES"].apply(calc_descriptors10) # 10 descriptors
df10 = pd.concat([df_raw, desc10], axis=1)
df10
| Compound Name | CAS | SMILES | Solubility_mol_per_L | pKa | Toxicity | Melting Point | Reactivity | Oxidation Site | MolWt | LogP | TPSA | NumRings | NumHAcceptors | NumHDonors | NumRotatableBonds | HeavyAtomCount | FractionCSP3 | NumAromaticRings | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3,4-dihydro-1H-isochromene | 493-05-0 | c1ccc2c(c1)CCOC2 | 0.103906 | 5.80 | non_toxic | 65.8 | 1 | 8,10 | 134.178 | 1.7593 | 9.23 | 2.0 | 1.0 | 0.0 | 0.0 | 10.0 | 0.333333 | 1.0 |
| 1 | 9H-fluorene | 86-73-7 | c1ccc2c(c1)Cc1ccccc1-2 | 0.010460 | 5.82 | toxic | 90.0 | 1 | 7 | 166.223 | 3.2578 | 0.00 | 3.0 | 0.0 | 0.0 | 0.0 | 13.0 | 0.076923 | 2.0 |
| 2 | 1,2,3,4-tetrahydronaphthalene | 119-64-2 | c1ccc2c(c1)CCCC2 | 0.020589 | 5.74 | toxic | 69.4 | 1 | 7,10 | 132.206 | 2.5654 | 0.00 | 2.0 | 0.0 | 0.0 | 0.0 | 10.0 | 0.400000 | 1.0 |
| 3 | ethylbenzene | 100-41-4 | CCc1ccccc1 | 0.048107 | 5.87 | non_toxic | 65.0 | 1 | 1,2 | 106.168 | 2.2490 | 0.00 | 1.0 | 0.0 | 0.0 | 1.0 | 8.0 | 0.250000 | 1.0 |
| 4 | cyclohexene | 110-83-8 | C1=CCCCC1 | 0.060688 | 5.66 | non_toxic | 96.4 | 1 | 3,6 | 82.146 | 2.1166 | 0.00 | 1.0 | 0.0 | 0.0 | 0.0 | 6.0 | 0.666667 | 0.0 |
| ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| 570 | 2-naphthalen-2-ylpropan-2-amine | 90299-04-0 | CC(C)(N)c1ccc2ccccc2c1 | 0.018990 | 10.04 | toxic | 121.5 | -1 | -1 | 185.270 | 3.0336 | 26.02 | 2.0 | 1.0 | 1.0 | 1.0 | 14.0 | 0.230769 | 2.0 |
| 571 | 1-bromo-4-(methylamino)anthracene-9,10-dione | 128-93-8 | CNc1ccc(Br)c2c1C(=O)c1ccccc1C2=O | 0.021590 | 7.81 | toxic | 154.0 | -1 | -1 | 316.154 | 3.2662 | 46.17 | 3.0 | 3.0 | 1.0 | 1.0 | 19.0 | 0.066667 | 2.0 |
| 572 | 1-[6-(dimethylamino)naphthalen-2-yl]prop-2-en-... | 86636-92-2 | C=CC(=O)c1ccc2cc(N(C)C)ccc2c1 | 0.017866 | 8.58 | toxic | 128.3 | -1 | -1 | 225.291 | 3.2745 | 20.31 | 2.0 | 2.0 | 0.0 | 3.0 | 17.0 | 0.133333 | 2.0 |
| 573 | 1,2-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-... | 34316-15-9 | COc1ccc2c(c[n+](C)c3c4cc5c(cc4ccc23)OCO5)c1OC | 0.016210 | 5.54 | toxic | 215.6 | -1 | -1 | 348.378 | 3.7166 | 40.80 | 5.0 | 4.0 | 0.0 | 2.0 | 26.0 | 0.190476 | 4.0 |
| 574 | dimethyl anthracene-1,8-dicarboxylate | 93655-34-6 | COC(=O)c1cccc2cc3cccc(C(=O)OC)c3cc12 | 0.016761 | 5.43 | toxic | 175.3 | -1 | -1 | 294.306 | 3.5662 | 52.60 | 3.0 | 4.0 | 0.0 | 2.0 | 22.0 | 0.111111 | 3.0 |
575 rows × 19 columns
In the previous lecture, we learned how to explore our dataset by plotting the distribution of molecular properties using histograms.
Another way is with a mask, which filters molecules by conditions like molecular weight, LogP, etc. Later in this class we’ll generate new molecules, so it’s helpful to see how many remain in our training set after applying these filters.
# New mask: MolWt between 100–400, LogP between -1 and 5
mask = (
(df10["MolWt"].between(100, 400)) &
(df10["LogP"].between(-1, 3)) )
# Apply sampling
df_small = df10[mask].copy().sample(min(500, mask.sum()), random_state=42)
df_small.shape
(276, 19)
2. Unsupervised recap with a tiny PCA#
We standardize 10D descriptors and compute a 2D PCA() for a quick map. Recall that PCA helps us reduce complexity while preserving the main variation in the data, making it easier to visualize patterns and clusters.
from sklearn.decomposition import PCA
feat_cols = ["MolWt","LogP","TPSA","NumRings","NumHAcceptors","NumHDonors",
"NumRotatableBonds","HeavyAtomCount","FractionCSP3","NumAromaticRings"]
X = df_small[feat_cols].to_numpy(dtype=float)
scaler = StandardScaler().fit(X)
Xz = scaler.transform(X)
pca = PCA(n_components=2).fit(Xz)
Zp = pca.transform(Xz)
print(f"Five Examples of molecules (coordinates): {Zp[:5]}")
plt.scatter(Zp[:,0], Zp[:,1], c=df_small["NumRings"], cmap="viridis", s=12, alpha=0.7)
plt.colorbar(label="NumRings")
plt.xlabel("PC1"); plt.ylabel("PC2"); plt.title("PCA on 10 descriptors")
plt.show()
Five Examples of molecules (coordinates): [[ 1.39032814 -0.29230696]
[ 4.45009367 -0.51704755]
[-0.50601149 -0.62038367]
[ 4.49210377 -0.02207216]
[-1.4659676 -0.55787823]]
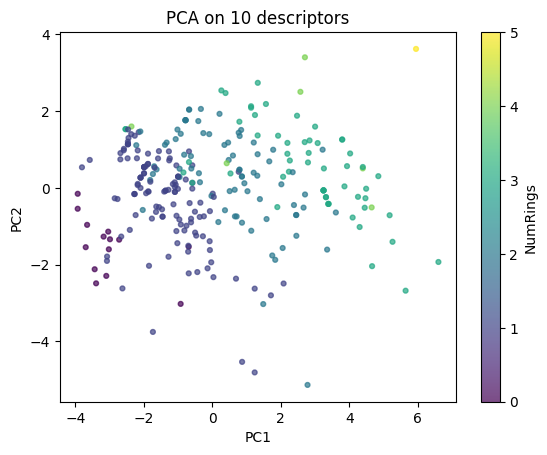
In the scatter plot we colored the points by the number of rings. This is not required every time, and you could just use a single color for all points. Adding a property as color is simply a way to help you better visualize patterns in the PCA map.
Below we look at loadings to see which descriptors drive PC1.
loadings = pd.Series(pca.components_[0], index=feat_cols).sort_values()
loadings
| 0 | |
|---|---|
| FractionCSP3 | -0.258801 |
| LogP | -0.012405 |
| NumRotatableBonds | -0.004420 |
| NumHDonors | 0.292818 |
| NumAromaticRings | 0.332956 |
| NumRings | 0.343686 |
| NumHAcceptors | 0.381308 |
| MolWt | 0.391912 |
| TPSA | 0.393411 |
| HeavyAtomCount | 0.405417 |
⏰ Exercise
Replace color by TPSA in the PCA scatter. What region corresponds to high TPSA?
#TO DO
3. Autoencoder on descriptors#
We will train a tiny autoencoder (AE) that learns a low-dimensional summary of our 10 standardized descriptors.
Let a molecule’s descriptor vector be: \( x \in \mathbb{R}^{10} \)
The encoder is a function \(f_\theta\) parameterized by weights \(\theta\). It maps the input \(x\) into a latent code \(z\):
\( z = f_\theta(x), \quad z \in \mathbb{R}^2 \)
The decoder is a function \(g_\phi\) parameterized by weights \(\phi\). It maps \(z\) back to a reconstructed vector \(\hat{x}\) in the original descriptor space:
\( \hat{x} = g_\phi(z), \quad \hat{x} \in \mathbb{R}^{10} \)
The training goal is to minimize the reconstruction loss, measured by the mean squared error (MSE) between the input and its reconstruction:
\( \mathcal{L}(\theta, \phi) \;=\; \frac{1}{N} \sum_{i=1}^N \lVert x_i - \hat{x}_i \rVert_2^2 \quad \text{with} \quad \hat{x}_i = g_\phi\!\big(f_\theta(x_i)\big). \)
The encoder acts like a compressor: it reduces the 10D descriptor into 2D latent space.
The decoder acts like an expander: it tries to reconstruct the original 10D input from the 2D code.
The loss function measures how close the reconstructed vector is to the original input.
By training the AE, we learn a latent space where molecules with similar properties may cluster together. Later, this latent space will be useful for generation, since we can sample points in the space and decode them into new molecular-like descriptors. Intuitively, the encoder compresses, the decoder unpacks, and the loss measures how faithful the unpacked vector is to the input.
Intuitively, the encoder compresses, the decoder unpacks, and the loss measures how faithful the unpacked vector is to the input.
We now implement a very small AE in PyTorch with one hidden layer of 8 units.
Our input has 10 features, this allows the encoder to pass through a slightly smaller hidden layer before reaching the bottleneck size (2D), which forces information to be distilled.
In other words, the encoder reduces 10 → 8 → 2, and the decoder reconstructs 2 → 8 → 10.
class TinyAE(nn.Module):
def __init__(self, in_dim=10, hid=8, z_dim=2):
super().__init__()
self.enc = nn.Sequential(nn.Linear(in_dim, hid), nn.ReLU(), nn.Linear(hid, z_dim))
self.dec = nn.Sequential(nn.Linear(z_dim, hid), nn.ReLU(), nn.Linear(hid, in_dim))
def encode(self, x): return self.enc(x)
def decode(self, z): return self.dec(z)
def forward(self, x):
z = self.enc(x)
xr = self.dec(z)
return xr, z
ae = TinyAE(in_dim=10, hid=8, z_dim=2)
ae
TinyAE(
(enc): Sequential(
(0): Linear(in_features=10, out_features=8, bias=True)
(1): ReLU()
(2): Linear(in_features=8, out_features=2, bias=True)
)
(dec): Sequential(
(0): Linear(in_features=2, out_features=8, bias=True)
(1): ReLU()
(2): Linear(in_features=8, out_features=10, bias=True)
)
)
We now wrap our standardized descriptors into a PyTorch dataset and build a DataLoader. The DataLoader controls how many samples are processed in each mini-batch.
Since our dataset has about 500 molecules, Batch size = 64 is a good choice.
class ArrayDataset(Dataset):
def __init__(self, X):
self.X = torch.from_numpy(X.astype(np.float32))
def __len__(self): return len(self.X)
def __getitem__(self, i): return self.X[i]
ds = ArrayDataset(Xz)
dl = DataLoader(ds, batch_size=64, shuffle=True)
xb = next(iter(dl))
xb.shape, xb[0,:4]
(torch.Size([64, 10]), tensor([ 0.7818, 0.1602, -0.4250, -0.7599]))
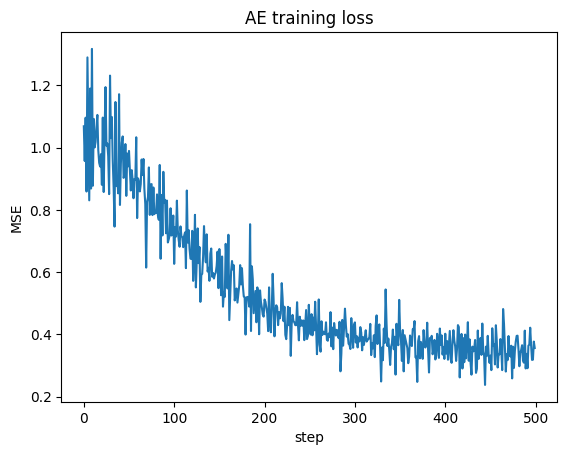
Train for a few epochs and watch the loss.
opt = optim.Adam(ae.parameters(), lr=1e-3)
losses = []
for ep in range(100):
for xb in dl:
xr, z = ae(xb)
loss = nn.functional.mse_loss(xr, xb)
opt.zero_grad(); loss.backward(); opt.step()
losses.append(loss.item())
plt.plot(losses); plt.xlabel("step"); plt.ylabel("MSE"); plt.title("AE training loss"); plt.show()
After training, we use the encoder to map all molecules into the 2D latent space. Each row of Z is a compressed representation of one molecule. This is what encode returns.
with torch.no_grad():
Z = ae.encode(torch.from_numpy(Xz.astype(np.float32))).numpy()
Z[:5]
array([[-0.03611878, -2.8281732 ],
[-2.0007432 , -4.8048697 ],
[ 0.91882634, -1.5941781 ],
[-1.969413 , -4.800315 ],
[ 1.3514404 , -0.10296482]], dtype=float32)
# Sample 3 random molecules
sample_df = df_small.sample(3, random_state=42)
# Get SMILES and mol objects
smiles_list = sample_df["SMILES"].tolist()
mol_list = [Chem.MolFromSmiles(s) for s in smiles_list]
# Encode the descriptors into latent space
with torch.no_grad():
sample_X = scaler.transform(sample_df[feat_cols].to_numpy(dtype=float))
Z_sample = ae.encode(torch.from_numpy(sample_X.astype(np.float32))).numpy()
# Draw molecules
img = Draw.MolsToGridImage(mol_list, molsPerRow=3, subImgSize=(200,200), legends=[f"SMILES: {s}" for s in smiles_list])
display(img)
# Print SMILES and encodings
for smi, z in zip(smiles_list, Z_sample):
print(f"SMILES: {smi}")
print(f"Encoded: {z}\n")
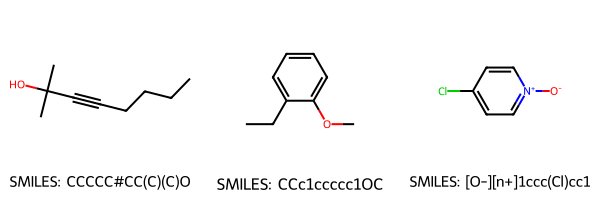
SMILES: CCCCC#CC(C)(C)O
Encoded: [ 3.5627599 -0.9266861]
SMILES: CCc1ccccc1OC
Encoded: [ 1.9100477 -0.19946669]
SMILES: [O-][n+]1ccc(Cl)cc1
Encoded: [ 1.4451884 -0.226154 ]
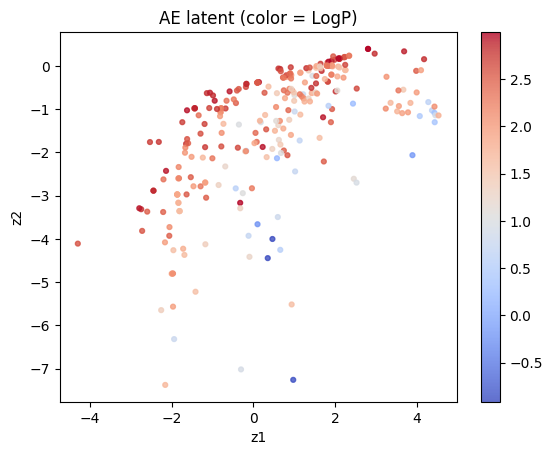
Plot the latent and color by LogP.
plt.scatter(Z[:,0], Z[:,1], c=df_small["LogP"], cmap="coolwarm", s=12, alpha=0.8)
plt.xlabel("z1"); plt.ylabel("z2"); plt.title("AE latent (color = LogP)")
plt.colorbar(); plt.show()
⏰ Exercise
Change z_dim to 3 in TinyAE and plot the latent. Do you see any difference?
Below we pick one random molecule to show after autoencoder compresses descriptors into latent space, it then reconstructs them back:
# Select one molecule
one_row = df_small.sample(1, random_state=7)
one_smiles = one_row["SMILES"].iloc[0]
one_mol = Chem.MolFromSmiles(one_smiles)
# Original descriptors (unscaled)
x_orig = one_row[feat_cols].to_numpy(dtype=float)
# Encode and decode (roundtrip)
with torch.no_grad():
x_std = scaler.transform(x_orig) # standardize
z = ae.encode(torch.from_numpy(x_std.astype(np.float32))).numpy() # latent code
x_rec_std = ae.decode(torch.from_numpy(z.astype(np.float32))).numpy()
x_rec = scaler.inverse_transform(x_rec_std) # back to original units
# Comparison table
df_compare = pd.DataFrame({
"Descriptor": feat_cols,
"Original": x_orig.flatten(),
"Reconstructed": x_rec.flatten()
})
df_compare["AbsError"] = np.abs(df_compare["Original"] - df_compare["Reconstructed"])
# Show molecule image
img = Draw.MolsToGridImage([one_mol], molsPerRow=1, subImgSize=(260,260), legends=[f"SMILES: {one_smiles}"])
display(img)
# Print latent vector
print("=== Roundtrip demonstration ===")
print(f"SMILES: {one_smiles}")
print(f"Latent z = ({z[0,0]:.4f}, {z[0,1]:.4f})\n")
# Display table
from IPython.display import display
display(df_compare.style.format({"Original": "{:.3f}", "Reconstructed": "{:.3f}", "AbsError": "{:.3f}"}))
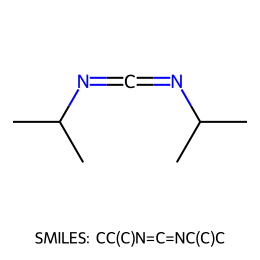
=== Roundtrip demonstration ===
SMILES: CC(C)N=C=NC(C)C
Latent z = (3.7859, -1.0718)
| Descriptor | Original | Reconstructed | AbsError | |
|---|---|---|---|---|
| 0 | MolWt | 126.203 | 126.923 | 0.720 |
| 1 | LogP | 1.977 | 1.455 | 0.522 |
| 2 | TPSA | 24.720 | 20.466 | 4.254 |
| 3 | NumRings | 0.000 | 0.587 | 0.587 |
| 4 | NumHAcceptors | 2.000 | 1.216 | 0.784 |
| 5 | NumHDonors | 0.000 | 0.132 | 0.132 |
| 6 | NumRotatableBonds | 2.000 | 1.621 | 0.379 |
| 7 | HeavyAtomCount | 9.000 | 8.381 | 0.619 |
| 8 | FractionCSP3 | 0.857 | 0.718 | 0.140 |
| 9 | NumAromaticRings | 0.000 | -0.054 | 0.054 |
In the above section, we saw that using a simple autoencoder on 10 descriptors with a very narrow bottleneck (10 → 8 → 2 → 8 → 10) generally did a fair job on reconstructions but not very good. This happens because descriptors are continuous, relatively few, and do not contain enough redundancy for the network to compress and expand reliably.
A better strategy for testing reconstruction is to use high-dimensional representations. These vectors typically give the autoencoder much richer structure to learn from.
Still, with limited data (~500) in our case it will not be perfect, but at least give you an idea of the improvement we can see:
from torch.utils.data import Dataset, DataLoader
from rdkit.Chem import AllChem, Draw
# 1) Build Morgan fingerprints from df_small["SMILES"]
def morgan_bits(smiles, nBits=512, radius=2):
m = Chem.MolFromSmiles(smiles)
bv = AllChem.GetMorganFingerprintAsBitVect(m, radius=radius, nBits=nBits)
arr = np.zeros((nBits,), dtype=np.int8)
Chem.DataStructs.ConvertToNumpyArray(bv, arr)
return arr
smiles_all = df_small["SMILES"].tolist()
X_bits = np.vstack([morgan_bits(s, 512, 2) for s in smiles_all]).astype(np.float32)
# 2) Dataset + DataLoader
class BitsetDS(Dataset):
def __init__(self, X): self.X = torch.from_numpy(X)
def __len__(self): return len(self.X)
def __getitem__(self, i): return self.X[i]
ds = BitsetDS(X_bits)
dl = DataLoader(ds, batch_size=64, shuffle=True)
# 3) AE with higher capacity; BCEWithLogitsLoss for binary recon
class MorganAE(nn.Module):
def __init__(self, in_dim=512, h1=256, h2=128, z_dim=32):
super().__init__()
self.enc = nn.Sequential(
nn.Linear(in_dim, h1), nn.ReLU(),
nn.Linear(h1, h2), nn.ReLU(),
nn.Linear(h2, z_dim)
)
self.dec = nn.Sequential(
nn.Linear(z_dim, h2), nn.ReLU(),
nn.Linear(h2, h1), nn.ReLU(),
nn.Linear(h1, in_dim) # logits
)
def encode(self, x): return self.enc(x)
def decode_logits(self, z): return self.dec(z) # logits
def forward(self, x):
z = self.encode(x)
logits = self.decode_logits(z)
return logits, z
mg_ae = MorganAE()
opt = optim.Adam(mg_ae.parameters(), lr=1e-3)
crit = nn.BCEWithLogitsLoss()
# 4) Train (few epochs already fit very well for 1024-bit vectors)
mg_ae.train()
for ep in range(100):
for xb in dl:
logits, _ = mg_ae(xb)
loss = crit(logits, xb)
opt.zero_grad(); loss.backward(); opt.step()
# 5) Pick one random molecule; show SMILES, Morgan bits, latent, reconstructed bits
idx = 102
smi = smiles_all[idx]
mol = Chem.MolFromSmiles(smi)
x_bits = torch.from_numpy(X_bits[idx:idx+1]) # shape (1, 1024)
mg_ae.eval()
with torch.no_grad():
z = mg_ae.encode(x_bits).numpy()[0] # latent vector
logits = mg_ae.decode_logits(torch.from_numpy(z[None, :]).float()) # logits
probs = torch.sigmoid(logits).numpy()[0] # probabilities in [0,1]
x_rec_bits = (probs >= 0.5).astype(np.float32) # thresholded reconstruction
acc = (x_rec_bits == X_bits[idx]).mean()
# 6) Display: molecule image, text summary; show first 64 bits for compact view
img = Draw.MolsToGridImage([mol], molsPerRow=1, subImgSize=(280, 280), legends=[f"SMILES: {smi}"])
display(img)
print("=== Random molecule roundtrip with Morgan-AE ===")
print(f"SMILES: {smi}")
print(f"Latent z (length {len(z)}): {np.round(z, 3)}")
print(f"Bit accuracy (full 512): {acc:.4f}")
print("\nFirst 64 original bits:\n", "".join(map(str, X_bits[idx, :100].astype(int))))
print("First 64 reconstructed bits:\n", "".join(map(str, x_rec_bits[:100].astype(int))))
# Also show a compact table with counts
orig_ones = int(X_bits[idx].sum())
rec_ones = int(x_rec_bits.sum())
agree_ones = int(((X_bits[idx] == 1) & (x_rec_bits == 1)).sum())
agree_zeros = int(((X_bits[idx] == 0) & (x_rec_bits == 0)).sum())
print("\nCounts:")
print(pd.DataFrame({
"metric": ["orig ones", "rec ones", "agree ones", "agree zeros", "total acc"],
"value": [orig_ones, rec_ones, agree_ones, agree_zeros, f"{acc:.4f}"]
}))
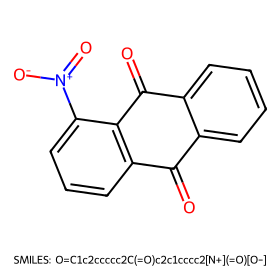
=== Random molecule roundtrip with Morgan-AE ===
SMILES: O=C1c2ccccc2C(=O)c2c1cccc2[N+](=O)[O-]
Latent z (length 32): [ 0.747 -3.635 5.305 -4.967 -1.144 5.502 3.814 6.794 -4.252 3.045
1.149 -1.759 0.103 -0.034 -2.485 -2.593 -6.326 3.881 -2.797 -3.996
-4.614 4.624 2.352 1.279 -4.574 3.405 2.413 -1.718 -1.09 4.06
4.541 4.271]
Bit accuracy (full 512): 0.9824
First 64 original bits:
0100000000000001000000000000000000000000000000000000000000000000100000000000000000000000000000000000
First 64 reconstructed bits:
0100000000000001000000000000000000000000000000000000000000000000100000000000000000000000000000000000
Counts:
metric value
0 orig ones 26
1 rec ones 25
2 agree ones 21
3 agree zeros 482
4 total acc 0.9824
Note that for the Morgan fingerprint autoencoder the input vectors are binary (0/1). In this case, we want the decoder to output logits that are turned into probabilities with a sigmoid. To measure the reconstruction, we use the binary cross-entropy loss (BCE), specifically BCEWithLogitsLoss in PyTorch.
The BCE loss is:
where:
\(x_{ij} \in \{0,1\}\) is the true fingerprint bit.
\(\hat{x}_{ij} \in \mathbb{R}\) is the decoder’s raw output (logit).
\(\sigma(\hat{x}_{ij}) = \tfrac{1}{1+e^{-\hat{x}_{ij}}}\) is the sigmoid that maps logits to probabilities.
While in previous case, the descriptor autoencoder (10 → 8 → 2 → 8 → 10) we minimized mean squared error (MSE) because the inputs were continuous-valued descriptors. The loss was: $\( \mathcal{L}_{\text{MSE}}(\theta,\phi) \;=\; \frac{1}{N}\sum_{i=1}^N \lVert x_i - \hat{x}_i \rVert_2^2 \)$
where \(x_i \in \mathbb{R}^{10}\) are real-valued molecular descriptors.
4. Why AE is tricky for SMILES#
While it is exciting to see the decoder can convert latent variable back to something similar to the input, it is important to point out a issue when it’s molecule generation:
an autoencoder that reconstructs descriptors or fingerprints does not guarantee that the reconstructed vector actually corresponds to a real molecule or a valid SMILES string.
With descriptors, the AE only learns to match numerical values (like MolWt, LogP, TPSA). A reconstructed descriptor vector might have numbers that do not correspond to any chemically valid structure. For example, a molecule cannot simultaneously have a negative molecular weight or a non-integer ring count.
With fingerprints, the AE tries to reconstruct binary patterns. A reconstructed bit vector might not map back to any actual molecule, since Morgan fingerprints are not bijective (different molecules can share fingerprints, and not every bit pattern corresponds to a valid molecule).
So even if the AE achieves a low reconstruction error, there is no guarantee that \(\hat{x}\) corresponds to a valid SMILES.
from torch.utils.data import TensorDataset, DataLoader
# --- assumes these exist from earlier cells ---
# df10, df_small, feat_cols, scaler, Xz
# 0) Discrete/continuous fields and tolerances (your originals)
DISCRETE = ["NumRings","NumHAcceptors","NumHDonors","NumRotatableBonds","HeavyAtomCount","NumAromaticRings"]
CONTINUOUS = [c for c in feat_cols if c not in DISCRETE]
TOL = {"MolWt": 2.0, "LogP": 0.2, "TPSA": 5.0, "FractionCSP3": 0.05}
def find_match_in_dataset(target: pd.Series, df_features: pd.DataFrame):
mask = np.ones(len(df_features), dtype=bool)
for d in DISCRETE:
mask &= (df_features[d].round().astype(int) == int(round(target[d])))
for c in CONTINUOUS:
tol = TOL.get(c, 0.5)
mask &= (np.abs(df_features[c] - target[c]) <= tol)
idx = np.where(mask)[0]
return idx
def nearest_neighbors(target_vec: np.ndarray, mat: np.ndarray, k=5):
d = np.linalg.norm(mat - target_vec[None, :], axis=1)
order = np.argsort(d)
return order[:k], d[order[:k]]
# 1) Define a descriptor AE separate from any Morgan AE
class TinyDescriptorAE(nn.Module):
def __init__(self, in_dim=10, hid=8, z_dim=2):
super().__init__()
self.enc = nn.Sequential(
nn.Linear(in_dim, hid), nn.ReLU(),
nn.Linear(hid, z_dim)
)
self.dec = nn.Sequential(
nn.Linear(z_dim, hid), nn.ReLU(),
nn.Linear(hid, in_dim)
)
def encode(self, x): return self.enc(x)
def decode(self, z): return self.dec(z)
def forward(self, x):
z = self.encode(x)
xr = self.decode(z)
return xr, z
# 2) Train desc_ae on standardized 10D descriptors
desc_ae = TinyDescriptorAE(in_dim=10, hid=8, z_dim=2)
dl = DataLoader(TensorDataset(torch.from_numpy(Xz.astype(np.float32))), batch_size=32, shuffle=True)
opt = optim.Adam(desc_ae.parameters(), lr=1e-3)
for ep in range(60): # 60 short epochs; increase if you want tighter recon
for (xb,) in dl:
xr, _ = desc_ae(xb)
loss = nn.functional.mse_loss(xr, xb)
opt.zero_grad(); loss.backward(); opt.step()
desc_ae.eval()
# 3) Pick one molecule and do encode -> decode with the CORRECT model (desc_ae)
one = df_small.sample(1, random_state=202)
one_smiles = one["SMILES"].iloc[0]
one_mol = Chem.MolFromSmiles(one_smiles)
x_orig = one[feat_cols].to_numpy(dtype=float) # shape (1, 10)
with torch.no_grad():
x_std = scaler.transform(x_orig).astype(np.float32) # standardize
z = desc_ae.encode(torch.from_numpy(x_std)).numpy() # latent via desc_ae
x_rec_std = desc_ae.decode(torch.from_numpy(z.astype(np.float32))).numpy()
x_rec = scaler.inverse_transform(x_rec_std)[0] # back to original units
# 4) Build a "constrained" target by rounding discrete fields and clipping bounds
target = pd.Series(x_rec, index=feat_cols)
for d in DISCRETE:
target[d] = int(round(float(target[d])))
target["FractionCSP3"] = float(np.clip(target["FractionCSP3"], 0.0, 1.0))
for cnt in DISCRETE:
target[cnt] = max(0, int(target[cnt]))
# 5) Search dataset for a feasible match under tolerances; else show nearest neighbors
df_feats_only = df10[feat_cols].copy()
matches = find_match_in_dataset(target, df_feats_only)
print("=== Attempt to invert descriptors to a molecule ===")
print(f"Original SMILES: {one_smiles}")
print(f"Latent z: {np.round(z[0], 4)}\n")
if len(matches) == 0:
print("No molecule in the dataset matches the reconstructed descriptor targets under tight tolerances.\n")
target_vec = target.values.astype(float)
mat = df_feats_only.to_numpy(dtype=float)
nn_idx, nn_dist = nearest_neighbors(target_vec, mat, k=5)
nn_rows = df10.iloc[nn_idx][["SMILES"] + feat_cols].copy()
nn_rows.insert(1, "distance", nn_dist)
display(nn_rows.head(5).style.format(precision=3))
# Draw original vs nearest by descriptors
top1_smiles = df10.iloc[nn_idx[0]]["SMILES"]
top1_mol = Chem.MolFromSmiles(top1_smiles)
img = Draw.MolsToGridImage([one_mol, top1_mol], molsPerRow=2, subImgSize=(260,260),
legends=[f"Original\n{one_smiles}",
f"Nearest by descriptors\n{top1_smiles}\nDist={nn_dist[0]:.3f}"])
display(img)
else:
print(f"Found {len(matches)} dataset candidate(s) matching targets under tolerances.")
display(df10.iloc[matches][["SMILES"] + feat_cols].head(5))
# 6) Compare original vs reconstructed target values
compare = pd.DataFrame({
"Descriptor": feat_cols,
"Original": x_orig.flatten(),
"Recon": [target[c] for c in feat_cols]
})
compare["AbsError"] = np.abs(compare["Original"] - compare["Recon"])
display(compare.style.format({"Original": "{:.3f}", "Recon": "{:.3f}", "AbsError": "{:.3f}"}))
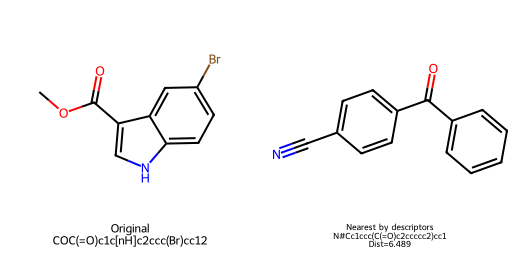
=== Attempt to invert descriptors to a molecule ===
Original SMILES: COC(=O)c1c[nH]c2ccc(Br)cc12
Latent z: [ 1.608 -0.5941]
No molecule in the dataset matches the reconstructed descriptor targets under tight tolerances.
| SMILES | distance | MolWt | LogP | TPSA | NumRings | NumHAcceptors | NumHDonors | NumRotatableBonds | HeavyAtomCount | FractionCSP3 | NumAromaticRings | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 497 | N#Cc1ccc(C(=O)c2ccccc2)cc1 | 6.489 | 207.232 | 2.789 | 40.860 | 2.000 | 2.000 | 0.000 | 2.000 | 16.000 | 0.000 | 2.000 |
| 79 | COC(=O)C1CCCCC1C(=O)OC | 8.741 | 200.234 | 1.139 | 52.600 | 1.000 | 4.000 | 0.000 | 2.000 | 14.000 | 0.800 | 0.000 |
| 564 | Nc1c(N)c2ccccc2c2ccccc12 | 9.238 | 208.264 | 3.157 | 52.040 | 3.000 | 2.000 | 2.000 | 0.000 | 16.000 | 0.000 | 3.000 |
| 243 | O=C(O)C(=O)c1ccc2ccccc2c1 | 9.653 | 200.193 | 2.107 | 54.370 | 2.000 | 2.000 | 1.000 | 2.000 | 15.000 | 0.000 | 2.000 |
| 378 | Nc1nc(-c2ccc(Cl)cc2)cs1 | 10.408 | 210.689 | 3.046 | 38.910 | 2.000 | 3.000 | 1.000 | 1.000 | 13.000 | 0.000 | 2.000 |
| Descriptor | Original | Recon | AbsError | |
|---|---|---|---|---|
| 0 | MolWt | 254.083 | 202.642 | 51.441 |
| 1 | LogP | 2.717 | 2.462 | 0.255 |
| 2 | TPSA | 42.090 | 45.094 | 3.004 |
| 3 | NumRings | 2.000 | 2.000 | 0.000 |
| 4 | NumHAcceptors | 2.000 | 2.000 | 0.000 |
| 5 | NumHDonors | 1.000 | 1.000 | 0.000 |
| 6 | NumRotatableBonds | 1.000 | 1.000 | 0.000 |
| 7 | HeavyAtomCount | 14.000 | 15.000 | 1.000 |
| 8 | FractionCSP3 | 0.100 | 0.088 | 0.012 |
| 9 | NumAromaticRings | 2.000 | 2.000 | 0.000 |
In the previous example we tried to decode reconstructed descriptors back to a molecule and saw that it often fails. The reconstructed values may look numerically close, yet they do not correspond to any real molecule.
With SMILES the situation becomes even more fragile. A single misplaced character is enough to make the entire string invalid. Unlike descriptors, which are continuous and can be perturbed slightly without losing “type”, SMILES is a discrete symbolic language with strict syntax rules. Parentheses must balance, ring indices must pair, and atom valences must be chemically possible.
The following short experiment takes valid SMILES, applies a single random character disturbance, and checks whether the result is still valid:
# Helpers: SMILES validity check and single-character perturbations
def is_valid_smiles(s: str) -> bool:
return Chem.MolFromSmiles(s) is not None
def random_char_edit(s: str, alphabet=None, p_insert=0.33, p_delete=0.33, p_sub=0.34):
if len(s) == 0: return s
if alphabet is None:
# Build a basic alphabet from common SMILES chars
alphabet = list(set(list("CNOFPSIclBr[#]=()1234567890+-@H[]\\/")))
r = random.random()
i = random.randrange(len(s))
if r < p_insert:
c = random.choice(alphabet)
return s[:i] + c + s[i:]
elif r < p_insert + p_delete and len(s) > 1:
return s[:i] + s[i+1:]
else:
c = random.choice(alphabet)
return s[:i] + c + s[i+1:]
# Experiment 1: one random edit kills validity most of the time
smiles_list = df_small["SMILES"].tolist()
k = min(200, len(smiles_list))
subset = random.sample(smiles_list, k)
perturbed = [random_char_edit(s) for s in subset]
valid_orig = sum(is_valid_smiles(s) for s in subset)
valid_pert = sum(is_valid_smiles(s) for s in perturbed)
print(f"Original valid: {valid_orig}/{k} = {valid_orig/k:.2%}")
print(f"After 1 random edit valid: {valid_pert}/{k} = {valid_pert/k:.2%}")
# Show a few examples
rows = []
for i in range(10):
s = subset[i]
t = perturbed[i]
rows.append({
"orig": s,
"perturbed": t,
"orig_valid": is_valid_smiles(s),
"perturbed_valid": is_valid_smiles(t)
})
pd.DataFrame(rows)
Original valid: 200/200 = 100.00%
After 1 random edit valid: 38/200 = 19.00%
| orig | perturbed | orig_valid | perturbed_valid | |
|---|---|---|---|---|
| 0 | COc1ccc(C(=O)O)cc1 | COc1ccc(C(=O))cc1 | True | True |
| 1 | Nc1ccccc1C(F)(F)F | Nc1cccrc1C(F)(F)F | True | False |
| 2 | CCOc1ccc(O)cc1 | CCOc1ccc(Occ1 | True | False |
| 3 | CC(C)Cc1ccccc1 | CC(C)Ccccccc1 | True | False |
| 4 | CCc1ccc(CC)cc1 | CCc1ccc-CC)cc1 | True | False |
| 5 | CCOC(=O)c1ccc(C#N)cc1 | CC3OC(=O)c1ccc(C#N)cc1 | True | False |
| 6 | Brc1ccc2[nH]ccc2c1 | Brc1ccc2[nH]ccc2cH1 | True | False |
| 7 | O=c1c(=O)c2cccc3ccc4cccc1c4c32 | =c1c(=O)c2cccc3ccc4cccc1c4c32 | True | False |
| 8 | CC1=C(C)C(=O)CCC1 | CHC1=C(C)C(=O)CCC1 | True | False |
| 9 | CN(C)C(n1n[n+]([O-])c2ncccc21)=[N+](C)C.F[P-](... | CN3(C)C(n1n[n+]([O-])c2ncccc21)=[N+](C)C.F[P-]... | True | False |
5. Variational Autoencoder (VAE)#
An AE compresses each input to a single point in latent space and learns to reconstruct that point. By constrast, a Variational Autoencoder (VAE) treats the latent code as a probability distribution. Instead of mapping an input to one vector, the encoder predicts a mean and a variance for a Gaussian latent. During training we sample from this Gaussian and ask the decoder to reconstruct the input from the sampled point. A Kullback–Leibler (KL) term softly pulls the posterior toward a simple prior such as a standard normal. The result is a latent space that is smoother and more continuous, which makes sampling new points more reliable.
This shift from point encoding to distribution encoding matters for generation. With a plain AE, latent space can be patchy. Interpolating between two codes can land you off the data manifold and the decoder struggles.
With a VAE, the KL term discourages such patchiness. The model learns a latent space where nearby points decode to similar objects, which helps when we draw new samples from the prior.
For SMILES generation, a well shaped latent space does not solve syntax by itself, but it does reduce the chance that sampling lands in regions that decode to garbage. In practice, VAEs pair well with more robust tokenizations or grammar constraints. Here we first build a compact VAE on the 10 standardized descriptors to make the idea concrete, then we show how to sample and decode new latent points.
Source: Link
import torch, torch.nn as nn, torch.optim as optim
class TinyVAE(nn.Module):
def __init__(self, in_dim=10, h=8, z_dim=2):
super().__init__()
# Encoder predicts mean and log-variance
self.enc = nn.Sequential(
nn.Linear(in_dim, h), nn.ReLU(),
nn.Linear(h, h), nn.ReLU()
)
self.mu = nn.Linear(h, z_dim)
self.logvar = nn.Linear(h, z_dim)
# Decoder maps z back to x
self.dec = nn.Sequential(
nn.Linear(z_dim, h), nn.ReLU(),
nn.Linear(h, h), nn.ReLU(),
nn.Linear(h, in_dim)
)
def encode(self, x):
h = self.enc(x)
return self.mu(h), self.logvar(h)
def reparameterize(self, mu, logvar):
std = torch.exp(0.5 * logvar)
eps = torch.randn_like(std)
return mu + eps * std
def decode(self, z):
return self.dec(z)
def forward(self, x):
mu, logvar = self.encode(x)
z = self.reparameterize(mu, logvar)
xr = self.decode(z)
return xr, mu, logvar, z
def vae_loss(xr, x, mu, logvar, beta=1.0):
# MSE recon + beta * KL
recon = nn.functional.mse_loss(xr, x, reduction='mean')
# KL for diagonal Gaussians: -0.5 * sum(1 + logvar - mu^2 - exp(logvar))
kl = -0.5 * torch.mean(1 + logvar - mu.pow(2) - logvar.exp())
return recon + beta * kl, recon.item(), kl.item()
# 1) Data loader
from torch.utils.data import DataLoader, TensorDataset
X_tensor = torch.from_numpy(Xz.astype(np.float32))
dl = DataLoader(TensorDataset(X_tensor), batch_size=128, shuffle=True)
# 2) Train
vae = TinyVAE(in_dim=10, h=8, z_dim=5)
opt = optim.Adam(vae.parameters(), lr=1e-3)
steps, recon_hist, kl_hist, loss_hist = [], [], [], []
global_step = 0
epochs = 2000
for ep in range(epochs):
# simple KL annealing from 0 -> 1 over training
beta = min(1.0, ep / max(1, epochs//2))
for (xb,) in dl:
xr, mu, logvar, z = vae(xb)
loss, r_item, k_item = vae_loss(xr, xb, mu, logvar, beta=beta)
opt.zero_grad(); loss.backward(); opt.step()
loss_hist.append(loss.item()); recon_hist.append(r_item); kl_hist.append(k_item)
steps.append(global_step); global_step += 1
# 3) Plot training curves
plt.figure()
plt.plot(loss_hist, label="total")
plt.plot(recon_hist, label="recon")
plt.plot(kl_hist, label="kl")
plt.legend(); plt.xlabel("step"); plt.ylabel("loss"); plt.title("VAE training")
plt.show()
# 4) Encode the dataset to latent means for visualization
vae.eval()
with torch.no_grad():
mu_all, logvar_all = vae.encode(X_tensor)
Z_mu = mu_all.numpy()
plt.figure()
plt.scatter(Z_mu[:,0], Z_mu[:,1], s=12, alpha=0.7)
plt.xlabel("z1"); plt.ylabel("z2"); plt.title("Latent means (VAE)")
plt.show()
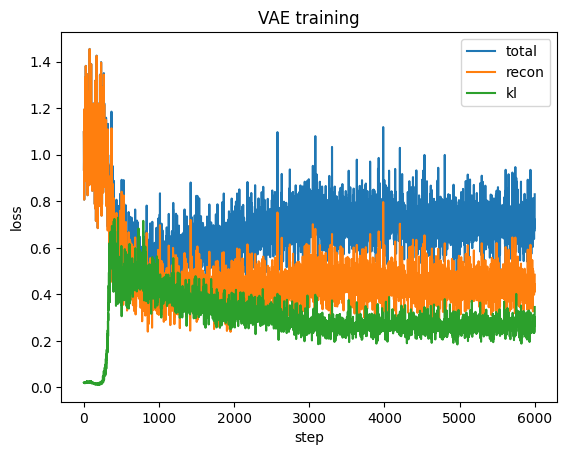
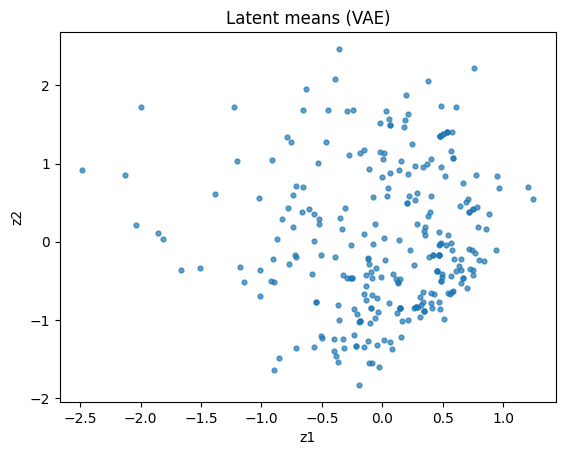
Note that in the example above the VAE is not very similar to Gaussian distribution because we only train a shallow one and our data size is limited.
The VAE maximizes the evidence lower bound (ELBO). In practice we minimize the negative ELBO, which has a reconstruction term and a KL term that regularizes the latent posterior toward the prior.
\( \mathcal{L}_{\text{VAE}} \;=\; \underbrace{\mathbb{E}_{q_\phi(z\mid x)}\big[\lVert x - \hat{x}\rVert_2^2\big]}_{\text{reconstruction}} \;+\; \beta\,\underbrace{D_{\text{KL}}\!\big(q_\phi(z\mid x)\;\|\;p(z)\big)}_{\text{regularization}} \,, \quad p(z)=\mathcal{N}(0,I). \)
To make sampling differentiable we use the reparameterization trick: \( z = \mu + \sigma \odot \epsilon,\quad \epsilon \sim \mathcal{N}(0, I),\quad \sigma = \exp\big(\tfrac{1}{2}\log\sigma^2\big). \)
# VAE distribution comparison for MolWt, LogP, TPSA + one-molecule demo
import numpy as np, pandas as pd, matplotlib.pyplot as plt
from rdkit.Chem import Draw
# Assumes these exist: vae (trained), scaler, df_small, feat_cols, Xz
# 1) Reconstruct the whole subset deterministically via latent means
vae.eval()
X_tensor = torch.from_numpy(Xz.astype(np.float32))
with torch.no_grad():
mu_all, logvar_all = vae.encode(X_tensor) # [N, zdim]
Xr_std = vae.decode(mu_all).numpy() # standardized recon
Xr = scaler.inverse_transform(Xr_std) # back to original units
Xr_df = pd.DataFrame(Xr, columns=feat_cols)
# Originals for comparison
orig_df = df_small[feat_cols].reset_index(drop=True)
# 2) Pick properties to compare
props = ["MolWt", "LogP", "TPSA"]
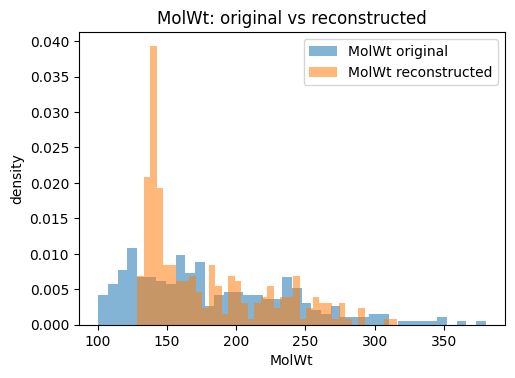
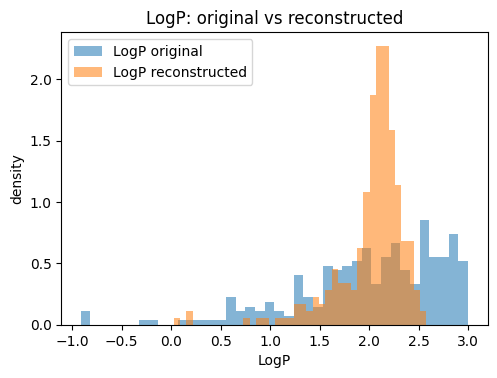
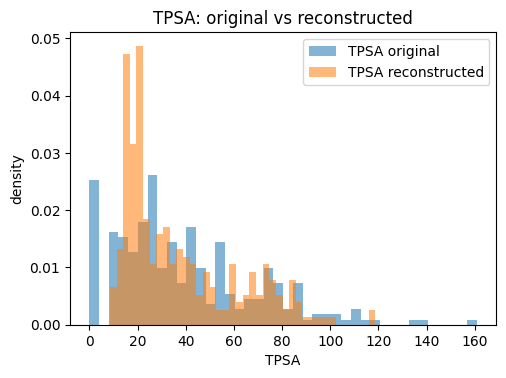
# 3) Histograms for each property (original vs reconstructed)
bins = 40
for p in props:
plt.figure(figsize=(5.5, 3.8))
plt.hist(orig_df[p].values, bins=bins, alpha=0.55, label=f"{p} original", density=True)
plt.hist(Xr_df[p].values, bins=bins, alpha=0.55, label=f"{p} reconstructed", density=True)
plt.xlabel(p); plt.ylabel("density"); plt.title(f"{p}: original vs reconstructed")
plt.legend()
plt.show()
# 4) Single random molecule: structure, latent mean, and 3-descriptor table
row = df_small.sample(1, random_state=314)
smi = row["SMILES"].iloc[0]
mol = Chem.MolFromSmiles(smi)
x_orig = row[feat_cols].to_numpy(dtype=float) # (1, 10)
with torch.no_grad():
x_std = scaler.transform(x_orig).astype(np.float32)
mu, logvar = vae.encode(torch.from_numpy(x_std))
z_mean = mu.numpy()[0] # latent mean (z-dim)
x_rec_std = vae.decode(mu).numpy() # decode from mean
x_rec = scaler.inverse_transform(x_rec_std) # (1, 10)
# Build a concise comparison table for the three properties
tbl = pd.DataFrame({
"Property": props,
"Original": [float(x_orig[0, feat_cols.index(p)]) for p in props],
"Reconstructed": [float(x_rec[0, feat_cols.index(p)]) for p in props]
})
tbl["AbsError"] = (tbl["Original"] - tbl["Reconstructed"]).abs()
# Show structure and print outputs
img = Draw.MolsToGridImage([mol], molsPerRow=1, subImgSize=(280, 280), legends=[f"SMILES: {smi}"])
display(img)
print("Latent mean z:", np.round(z_mean, 4))
display(tbl.style.format({"Original": "{:.3f}", "Reconstructed": "{:.3f}", "AbsError": "{:.3f}"}))
Latent mean z: [-0.1382 -0.4265 -0.4618 -0.0113 -0.0008]
| Property | Original | Reconstructed | AbsError | |
|---|---|---|---|---|
| 0 | MolWt | 189.258 | 148.060 | 41.198 |
| 1 | LogP | 2.313 | 2.179 | 0.134 |
| 2 | TPSA | 20.310 | 18.879 | 1.431 |
The reconstruction table typically shows that continuous descriptors such as molecular weight or TPSA are approximated closely, while discrete counts like ring numbers may drift toward fractional values. This is expected because the VAE optimizes for mean squared error, not for integer constraints. The key point is that the VAE provides a usable latent representation where small moves in latent space correspond to gradual changes in descriptors, making it much better suited for generation than a plain AE.
6. SMILES VAE for De Novo Molecular Generation#
In the previous examples, we are limited by the size of training data and the model complexicity so in general the performance is not perfect. Now, we will train a small SMILES-based Variational Autoencoder (VAE) on ~4,000 molecules, then sample new molecules and evaluate how well they match the training set.
Below are the steps:
Load a molecular dataset with DeepChem (QM9 subset)
Build a simple SMILES vocabulary
Train a GRU VAE for 10-30 epochs and plot loss
Generate new SMILES and filter invalid ones
Evaluate validity, uniqueness, novelty
Compare distributions of QED, logP, and molecular weight between train and generated sets
# Load QM9 via DeepChem (will download the dataset on first run)
tasks, datasets, transformers = dc.molnet.load_qm9(featurizer='Raw')
train_dataset, valid_dataset, test_dataset = datasets
def canonicalize_smiles(smi):
"""Return a canonical SMILES if valid, else None."""
if not smi:
return None
try:
# Parse with sanitize=False then sanitize manually to catch errors cleanly
m = Chem.MolFromSmiles(smi, sanitize=False)
if m is None:
return None
Chem.SanitizeMol(m)
return Chem.MolToSmiles(m, canonical=True)
except Exception:
return None
def dataset_to_smiles(ds, max_n=None):
"""Extract canonical SMILES from a DeepChem dataset of RDKit mols with progress updates."""
out = []
n = len(ds.X) if max_n is None else min(len(ds.X), max_n)
step = max(1, n // 10) # every 10%
for i in range(n):
mol = ds.X[i]
if mol is not None:
try:
smi = Chem.MolToSmiles(mol, canonical=True)
can = canonicalize_smiles(smi)
if can:
out.append(can)
except Exception:
continue
if (i + 1) % step == 0 or i == n - 1:
pct = int(((i + 1) / n) * 100)
print(f"Progress: {pct}% ({i + 1}/{n})")
return out
# Collect a pool then de-duplicate
pool_smiles = dataset_to_smiles(train_dataset, max_n=4000)
pool_smiles = list(dict.fromkeys(pool_smiles)) # keep order, remove duplicates
print("Pool size:", len(pool_smiles))
# If pool is smaller than 4K in your runtime, this will just take what's available
target_n = 4000
if len(pool_smiles) > target_n:
rng = np.random.default_rng(SEED)
smiles_all = rng.choice(pool_smiles, size=target_n, replace=False).tolist()
else:
smiles_all = pool_smiles[:target_n]
print("Training pool size used:", len(smiles_all))
print("Sample:", smiles_all[:5])
Progress: 10% (400/4000)
Progress: 20% (800/4000)
Progress: 30% (1200/4000)
Progress: 40% (1600/4000)
Progress: 50% (2000/4000)
Progress: 60% (2400/4000)
Progress: 70% (2800/4000)
Progress: 80% (3200/4000)
Progress: 90% (3600/4000)
Progress: 100% (4000/4000)
Pool size: 3999
Training pool size used: 3999
Sample: ['[H]O[C@@]1([H])C([H])([H])[N@H+]2C([H])([H])[C@@]13OC([H])([H])[C@@]23[H]', '[H]C([H])([H])[NH+](C([H])([H])[H])[C@@]12C([H])([H])O[C@]1([H])C2([H])[H]', '[H]C#CC([H])([H])OC12C([H])([H])[NH+](C1([H])[H])C2([H])[H]', '[H]N([H])c1c(C([H])([H])[H])noc1N([H])C([H])([H])[H]', '[H]C([H])(C#N)[NH2+][C@@H]1O[C@@]2([H])C([H])([H])[C@@]12[H]']
Note:
If above takes too long to write, change max_n and target_n to 2000 or 1200.
Train and validation split
train_smiles, val_smiles = train_test_split(smiles_all, test_size=0.1, random_state=SEED)
len(train_smiles), len(val_smiles)
(3599, 400)
We will build a simple character-level vocabulary. The model predicts the next character given the previous ones.
SPECIAL = ["[PAD]", "[SOS]", "[EOS]"]
def build_vocab(smiles_list):
chars = set()
for s in smiles_list:
for ch in s:
chars.add(ch)
idx2ch = SPECIAL + sorted(chars)
ch2idx = {c:i for i,c in enumerate(idx2ch)}
return ch2idx, idx2ch
ch2idx, idx2ch = build_vocab(train_smiles)
PAD, SOS, EOS = ch2idx["[PAD]"], ch2idx["[SOS]"], ch2idx["[EOS]"]
vocab_size = len(idx2ch)
MAX_LEN = 120 # raise if many strings are longer
def smiles_to_idx(s):
toks = [SOS] + [ch2idx[ch] for ch in s if ch in ch2idx] + [EOS]
toks = toks[:MAX_LEN]
attn = [1]*len(toks)
if len(toks) < MAX_LEN:
toks += [PAD]*(MAX_LEN - len(toks))
attn += [0]*(MAX_LEN - len(attn))
return np.array(toks, dtype=np.int64), np.array(attn, dtype=np.int64)
class SmilesDataset(Dataset):
def __init__(self, smiles_list):
enc = [smiles_to_idx(s) for s in smiles_list]
self.toks = np.stack([e[0] for e in enc])
self.attn = np.stack([e[1] for e in enc])
def __len__(self):
return len(self.toks)
def __getitem__(self, idx):
return torch.from_numpy(self.toks[idx]), torch.from_numpy(self.attn[idx])
train_ds = SmilesDataset(train_smiles)
val_ds = SmilesDataset(val_smiles)
train_loader = DataLoader(train_ds, batch_size=128, shuffle=True, drop_last=True)
val_loader = DataLoader(val_ds, batch_size=128, shuffle=False, drop_last=False)
print("Vocab size:", vocab_size, "Train size:", len(train_ds), "Val size:", len(val_ds))
print("Index to char sample:", idx2ch[:40])
Vocab size: 28 Train size: 3599 Val size: 400
Index to char sample: ['[PAD]', '[SOS]', '[EOS]', '#', '(', ')', '+', '-', '.', '/', '1', '2', '3', '4', '5', '=', '@', 'C', 'F', 'H', 'N', 'O', '[', '\\', ']', 'c', 'n', 'o']
Now, we will define a tiny SMILES VAE (GRU encoder and decoder), which is a compact model that trains quickly:
Embedding
GRU encoder produces mean and log-variance for latent vector
GRU decoder generates characters
Loss = cross entropy + KL term
class Encoder(nn.Module):
def __init__(self, vocab_size, emb_dim=128, hid_dim=256, z_dim=64):
super().__init__()
self.emb = nn.Embedding(vocab_size, emb_dim, padding_idx=PAD)
self.gru = nn.GRU(emb_dim, hid_dim, batch_first=True)
self.mu = nn.Linear(hid_dim, z_dim)
self.logvar = nn.Linear(hid_dim, z_dim)
def forward(self, x, attn):
emb = self.emb(x)
lengths = attn.sum(1).cpu()
packed = nn.utils.rnn.pack_padded_sequence(emb, lengths, batch_first=True, enforce_sorted=False)
_, h = self.gru(packed)
h = h[-1]
return self.mu(h), self.logvar(h)
class Decoder(nn.Module):
def __init__(self, vocab_size, emb_dim=128, hid_dim=256, z_dim=64):
super().__init__()
self.emb = nn.Embedding(vocab_size, emb_dim, padding_idx=PAD)
self.fc_z = nn.Linear(z_dim, hid_dim)
self.gru = nn.GRU(emb_dim, hid_dim, batch_first=True)
self.out = nn.Linear(hid_dim, vocab_size)
def forward(self, z, x_in):
h0 = self.fc_z(z).unsqueeze(0)
emb = self.emb(x_in)
o, _ = self.gru(emb, h0)
return self.out(o)
class VAE(nn.Module):
def __init__(self, vocab_size, emb_dim=128, hid_dim=256, z_dim=64):
super().__init__()
self.enc = Encoder(vocab_size, emb_dim, hid_dim, z_dim)
self.dec = Decoder(vocab_size, emb_dim, hid_dim, z_dim)
def reparameterize(self, mu, logvar):
std = torch.exp(0.5*logvar)
eps = torch.randn_like(std)
return mu + eps*std
def forward(self, x, attn):
mu, logvar = self.enc(x, attn)
z = self.reparameterize(mu, logvar)
logits = self.dec(z, x[:, :-1]) # teacher forcing
return logits, mu, logvar
def vae_loss(logits, x, mu, logvar, kl_weight=0.1):
targets = x[:, 1:]
ce = nn.functional.cross_entropy(logits.reshape(-1, logits.size(-1)),
targets.reshape(-1),
ignore_index=PAD)
kl = -0.5 * torch.mean(1 + logvar - mu.pow(2) - logvar.exp())
return ce + kl_weight*kl, ce.item(), kl.item()
model = VAE(vocab_size).to(device)
opt = optim.Adam(model.parameters(), lr=2e-3)
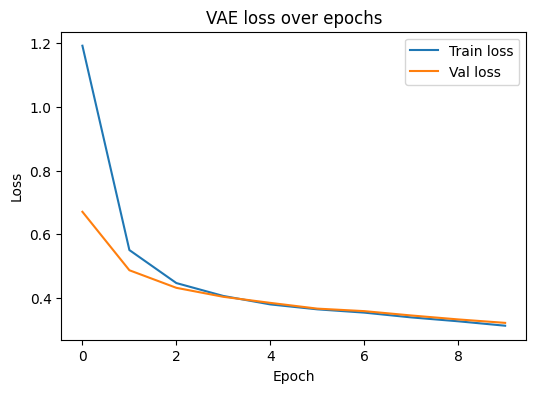
We track both training and validation loss. Lower is better. If validation loss stops improving, consider lowering learning rate or adding early stopping.
EPOCHS = 10
hist = {"train": [], "val": [], "ce": [], "kl": []}
for ep in range(1, EPOCHS+1):
model.train()
train_losses = []
ce_losses = []
kl_losses = []
for x, a in train_loader:
x, a = x.to(device), a.to(device)
opt.zero_grad()
logits, mu, logvar = model(x, a)
loss, ce, kl = vae_loss(logits, x, mu, logvar, kl_weight=0.1)
loss.backward()
nn.utils.clip_grad_norm_(model.parameters(), 1.0)
opt.step()
train_losses.append(loss.item())
ce_losses.append(ce)
kl_losses.append(kl)
model.eval()
with torch.no_grad():
val_losses = []
for x, a in val_loader:
x, a = x.to(device), a.to(device)
logits, mu, logvar = model(x, a)
l, _, _ = vae_loss(logits, x, mu, logvar, kl_weight=0.1)
val_losses.append(l.item())
tr = float(np.mean(train_losses))
va = float(np.mean(val_losses)) if len(val_losses) > 0 else float('nan')
hist["train"].append(tr)
hist["val"].append(va)
hist["ce"].append(float(np.mean(ce_losses)))
hist["kl"].append(float(np.mean(kl_losses)))
print(f"Epoch {ep:02d} | train {tr:.3f} | val {va:.3f} | CE {np.mean(ce_losses):.3f} | KL {np.mean(kl_losses):.3f}")
# Plot training and validation loss
plt.figure(figsize=(6,4))
plt.plot(hist["train"], label="Train loss")
plt.plot(hist["val"], label="Val loss")
plt.xlabel("Epoch")
plt.ylabel("Loss")
plt.title("VAE loss over epochs")
plt.legend()
plt.show()
Epoch 01 | train 1.193 | val 0.671 | CE 1.186 | KL 0.067
Epoch 02 | train 0.550 | val 0.487 | CE 0.546 | KL 0.046
Epoch 03 | train 0.446 | val 0.431 | CE 0.443 | KL 0.029
Epoch 04 | train 0.406 | val 0.403 | CE 0.404 | KL 0.020
Epoch 05 | train 0.379 | val 0.384 | CE 0.378 | KL 0.015
Epoch 06 | train 0.364 | val 0.366 | CE 0.362 | KL 0.015
Epoch 07 | train 0.353 | val 0.358 | CE 0.351 | KL 0.023
Epoch 08 | train 0.338 | val 0.345 | CE 0.335 | KL 0.035
Epoch 09 | train 0.326 | val 0.332 | CE 0.321 | KL 0.049
Epoch 10 | train 0.312 | val 0.321 | CE 0.305 | KL 0.068
Finally, we carry out sampling and evaluation.
Sample strings from the decoder by drawing z from the prior.
def sample_smiles(n=1500, max_len=120, temp=1.0):
model.eval()
out = []
with torch.no_grad():
z = torch.randn(n, model.enc.mu.out_features, device=device)
x_t = torch.full((n,1), SOS, dtype=torch.long, device=device)
h = model.dec.fc_z(z).unsqueeze(0)
for t in range(max_len-1):
emb = model.dec.emb(x_t[:,-1:])
o, h = model.dec.gru(emb, h)
logits = model.dec.out(o[:, -1])
probs = nn.functional.softmax(logits / temp, dim=-1)
nxt = torch.multinomial(probs, num_samples=1)
x_t = torch.cat([x_t, nxt], dim=1)
seqs = x_t[:, 1:].tolist()
for seq in seqs:
chars = []
for idx in seq:
ch = idx2ch[idx]
if ch == "[EOS]":
break
if ch not in ("[PAD]", "[SOS]"):
chars.append(ch)
out.append("".join(chars))
return out
def safe_mol_from_smiles(smi):
if not smi:
return None
try:
m = Chem.MolFromSmiles(smi, sanitize=False)
if m is None:
return None
Chem.SanitizeMol(m)
return m
except Exception:
return None
def canonicalize_batch(smiles_list):
out = []
for s in smiles_list:
m = safe_mol_from_smiles(s)
if m is None:
continue
can = Chem.MolToSmiles(m, canonical=True)
if can:
out.append(can)
return out
gen_raw = sample_smiles(n=2000, temp=1.3) # adjust temperature if desired
gen_smiles = canonicalize_batch(gen_raw)
print("Generated raw:", len(gen_raw), "Valid after sanitize:", len(gen_smiles))
Generated raw: 2000 Valid after sanitize: 230
train_set = set(train_smiles)
validity = len(gen_smiles) / max(1, len(gen_raw))
uniq_list = list(dict.fromkeys(gen_smiles))
uniqueness = len(uniq_list) / max(1, len(gen_smiles))
novelty = sum(1 for s in uniq_list if s not in train_set) / max(1, len(uniq_list))
print(f"Validity: {validity:.2f}")
print(f"Uniqueness: {uniqueness:.2f}")
print(f"Novelty: {novelty:.2f}")
Validity: 0.12
Uniqueness: 0.96
Novelty: 0.99
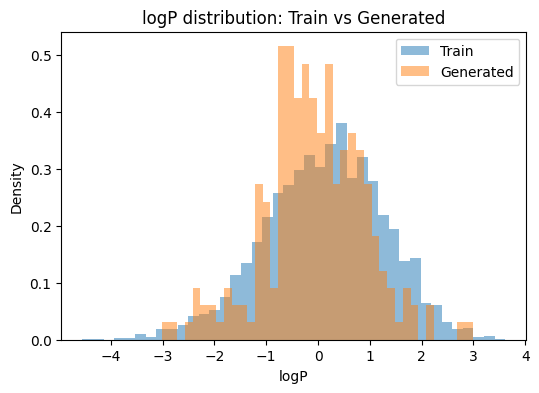
We compare QED, logP, and molecular weight between training and generated sets. If the model captured the training distribution, the histograms should overlap.
def props_from_smiles(smiles):
rows = []
for s in smiles:
m = safe_mol_from_smiles(s)
if m is None:
continue
try:
rows.append({
"SMILES": s,
"QED": QED.qed(m),
"logP": Crippen.MolLogP(m),
"MW": Descriptors.MolWt(m)
})
except Exception:
continue
return pd.DataFrame(rows)
# Subsample for quick plotting if needed
rng = np.random.default_rng(0)
train_unique = list(set(train_smiles))
train_sample = rng.choice(train_unique, size=min(3000, len(train_unique)), replace=False)
gen_unique = list(set(gen_smiles))
gen_sample = rng.choice(gen_unique, size=min(3000, len(gen_unique)), replace=False)
df_train = props_from_smiles(train_sample)
df_gen = props_from_smiles(gen_sample)
print("Train rows:", len(df_train), "Generated rows:", len(df_gen))
# Plot distributions (one metric per plot)
def plot_dist(metric, bins=40):
plt.figure(figsize=(6,4))
plt.hist(df_train[metric].dropna(), bins=bins, alpha=0.5, density=True, label="Train")
plt.hist(df_gen[metric].dropna(), bins=bins, alpha=0.5, density=True, label="Generated")
plt.xlabel(metric); plt.ylabel("Density")
plt.title(f"{metric} distribution: Train vs Generated")
plt.legend()
plt.show()
for m in ["QED", "logP", "MW"]:
plot_dist(m)
# Simple numeric distance summary with Wasserstein distance (optional)
try:
from scipy.stats import wasserstein_distance
for m in ["QED","logP","MW"]:
a = df_train[m].dropna().values
b = df_gen[m].dropna().values
w = wasserstein_distance(a, b) if len(a) > 10 and len(b) > 10 else float('nan')
print(f"{m} Wasserstein distance: {w:.4f}")
except Exception as e:
print("SciPy not available for Wasserstein distance. Skipping. Error:", e)
Train rows: 3000 Generated rows: 220
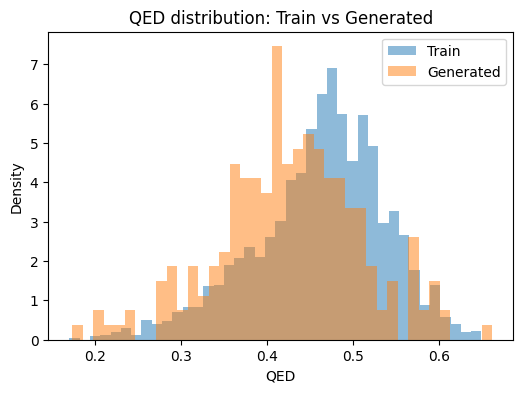
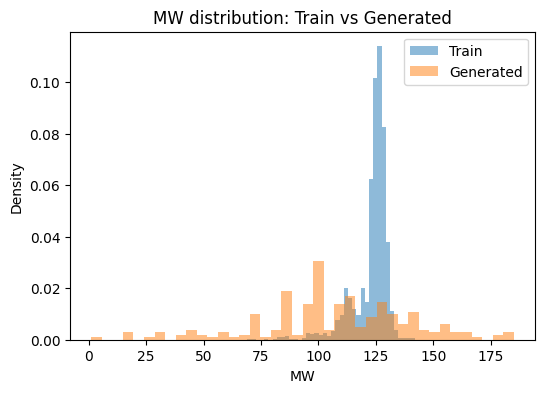
QED Wasserstein distance: 0.0352
logP Wasserstein distance: 0.2554
MW Wasserstein distance: 24.9263
⏰ Exercise
Run sample_smiles with temperatures 0.7 and 1.3. Which one increases validity? Which one increases uniqueness? How do the histograms shift?
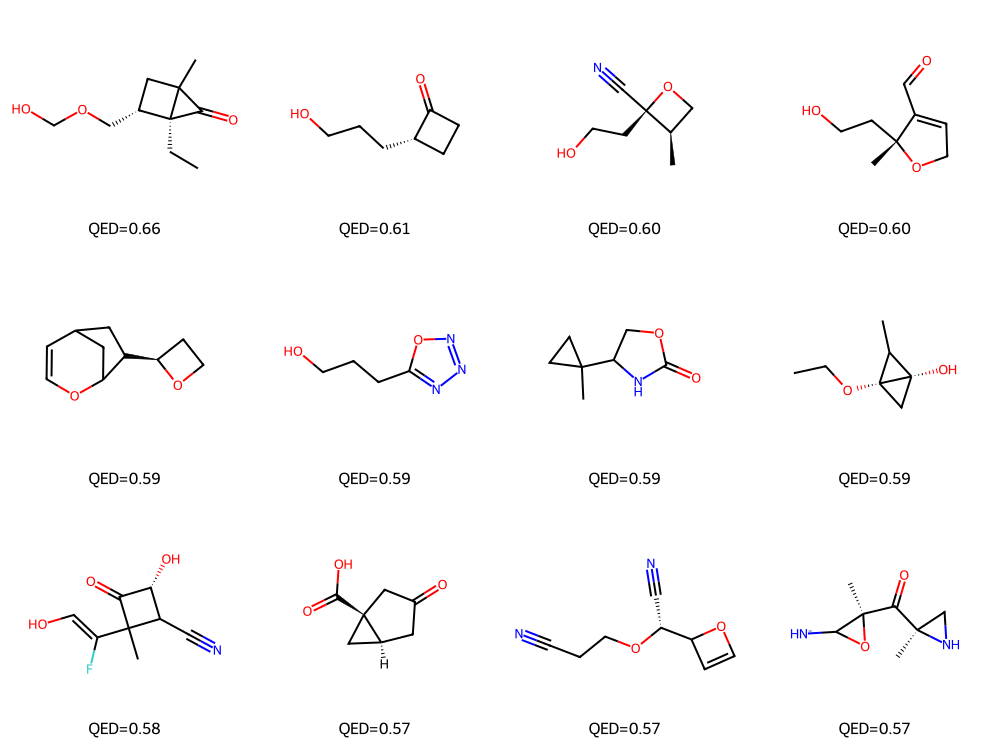
# Show a grid of top-QED generated molecules
df_gen_sorted = df_gen.sort_values(by="QED", ascending=False).reset_index(drop=True)
top_smiles = df_gen_sorted["SMILES"].head(12).tolist()
mols = [Chem.MolFromSmiles(s) for s in top_smiles]
img = Draw.MolsToGridImage(mols, molsPerRow=4, subImgSize=(250,250),
legends=[f"QED={q:.2f}" for q in df_gen_sorted["QED"].head(12)])
display(img)
7. Glossary#
encoder
A mapping from input \(x\) to latent \(z\).
decoder
A mapping from latent \(z\) to reconstructed \(\hat x\).
autoencoder (AE)
A model trained to reconstruct input. Learns a compact latent code.
latent space
The internal coordinate used by the model to organize inputs.
VAE
A probabilistic AE that learns \(q_\theta(z\mid x)\) near a simple prior to enable sampling.
validity
Fraction of generated strings that sanitize as molecules.
uniqueness
Fraction of valid generated molecules that are unique.
novelty
Fraction of unique generated molecules not present in the training set.
8. In-class activity#
Q1. AE latent: expand to 3D and inspect pairs#
For TinyAE() we defined before on Section 3, compare how the 2D views of a 3D latent organize molecules.
Steps
Write you own
TinyAE(). Instead of 8 nodes in hidden layer, try64(even though we use 10D descriptor, it’s still “reducing” the dimension because our bottle neck latent space is smaller 10)Replace the 2D latent with a 3D latent by setting
z_dim=3.Train the model for 10 epochs on
Xzusing MSE.Encode the dataset to get
Z3with shape[N, 3].Plot a 3D scatter with Matplotlib. Use
projection="3d". Label axesz0, z1, z2.
# TODO: your code here
from torch.utils.data import TensorDataset, DataLoader
dl_desc = DataLoader(
TensorDataset(torch.from_numpy(Xz.astype(np.float32))),
batch_size=64,
shuffle=True,
)
# 3D latent AE and training loop (unpack with (xb,))
ae3 = TinyAE(in_dim=10, hid=64, z_dim=3)
opt = optim.Adam(ae3.parameters(), lr=1e-3)
for ep in range(4):
for (xb,) in dl_desc:
xr, z = ae3(xb)
loss = nn.functional.mse_loss(xr, xb)
opt.zero_grad(); loss.backward(); opt.step()
# Encode with the trained model
with torch.no_grad():
Z3 = ae3.encode(torch.from_numpy(Xz.astype(np.float32))).numpy()
fig = plt.figure(figsize=(6, 5))
ax = fig.add_subplot(111, projection="3d")
p = ax.scatter(
Z3[:, 0], Z3[:, 1], Z3[:, 2],
c=df_small["LogP"].values,
s=12, alpha=0.85
)
ax.set_xlabel("z0"); ax.set_ylabel("z1"); ax.set_zlabel("z2")
ax.set_title("AE latent space (3D), color = LogP")
cb = fig.colorbar(p, ax=ax, shrink=0.7, pad=0.1)
cb.set_label("LogP")
plt.show()
Q2. VAE temperature sweep: validity vs sampling temperature#
Quantify how sampling temperature affects validity.
Steps
Use the provided
sample_smilesfunction.Set
n=800and testtempin[0.01, 0.1, 0.3, 0.5, 1.0, 1.5, 2]without changing other code.For each temperature, compute the fraction of valid canonical SMILES using
canonicalize_batch.Try to interprete the trade-off between conservativeness and diversity.
# TODO: your code here
for t in [0.01, 0.1, 0.3, 0.5, 1.0, 1.5, 2]:
raw = sample_smiles(n=800, temp=t)
val = len(canonicalize_batch(raw)) / max(1, len(raw))
print(f"T={t}: validity {val:.2f}")

