Lecture 11 - Dimension Reduction for Data Visualization#
Learning goals#
Understand unsupervised learning vs supervised learning in chemistry.
Explain the intuition and math of PCA and read loadings, scores, and explained variance.
Use t-SNE and UMAP to embed high dimensional chemical features to 2D for visualization.
1. Setup and data#
We will reuse the C-H oxidation dataset and compute a small set of descriptors.
url = "https://raw.githubusercontent.com/zzhenglab/ai4chem/main/book/_data/C_H_oxidation_dataset.csv"
df_raw = pd.read_csv(url)
df_raw.head(3)
| Compound Name | CAS | SMILES | Solubility_mol_per_L | pKa | Toxicity | Melting Point | Reactivity | Oxidation Site | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3,4-dihydro-1H-isochromene | 493-05-0 | c1ccc2c(c1)CCOC2 | 0.103906 | 5.80 | non_toxic | 65.8 | 1 | 8,10 |
| 1 | 9H-fluorene | 86-73-7 | c1ccc2c(c1)Cc1ccccc1-2 | 0.010460 | 5.82 | toxic | 90.0 | 1 | 7 |
| 2 | 1,2,3,4-tetrahydronaphthalene | 119-64-2 | c1ccc2c(c1)CCCC2 | 0.020589 | 5.74 | toxic | 69.4 | 1 | 7,10 |
We compute four quick 4 descriptors for everyone. Different from we we did previously, we only have another function compute 10 descriptors.
# Original function (4 descriptors)
def calc_descriptors(smiles: str):
if smiles is None:
return pd.Series({"MolWt": np.nan, "LogP": np.nan, "TPSA": np.nan, "NumRings": np.nan})
m = Chem.MolFromSmiles(smiles)
if m is None:
return pd.Series({"MolWt": np.nan, "LogP": np.nan, "TPSA": np.nan, "NumRings": np.nan})
return pd.Series({
"MolWt": Descriptors.MolWt(m),
"LogP": Crippen.MolLogP(m),
"TPSA": rdMolDescriptors.CalcTPSA(m),
"NumRings": rdMolDescriptors.CalcNumRings(m),
})
# New function (10 descriptors)
def calc_descriptors10(smiles: str):
m = Chem.MolFromSmiles(smiles)
return pd.Series({
"MolWt": Descriptors.MolWt(m),
"LogP": Crippen.MolLogP(m),
"TPSA": rdMolDescriptors.CalcTPSA(m),
"NumRings": rdMolDescriptors.CalcNumRings(m),
"NumHAcceptors": rdMolDescriptors.CalcNumHBA(m),
"NumHDonors": rdMolDescriptors.CalcNumHBD(m),
"NumRotatableBonds": rdMolDescriptors.CalcNumRotatableBonds(m),
"HeavyAtomCount": Descriptors.HeavyAtomCount(m), # <-- fixed
"FractionCSP3": rdMolDescriptors.CalcFractionCSP3(m),
"NumAromaticRings": rdMolDescriptors.CalcNumAromaticRings(m)
})
# Example usage (choose which function you want to apply)
desc4 = df_raw["SMILES"].apply(calc_descriptors) # 4 descriptors
desc10 = df_raw["SMILES"].apply(calc_descriptors10) # 10 descriptors
df4 = pd.concat([df_raw, desc4], axis=1)
df10 = pd.concat([df_raw, desc10], axis=1)
print("Rows x Cols (4 desc):", df4.shape)
print("Rows x Cols (10 desc):", df10.shape)
df4.head()
Rows x Cols (4 desc): (575, 13)
Rows x Cols (10 desc): (575, 19)
| Compound Name | CAS | SMILES | Solubility_mol_per_L | pKa | Toxicity | Melting Point | Reactivity | Oxidation Site | MolWt | LogP | TPSA | NumRings | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3,4-dihydro-1H-isochromene | 493-05-0 | c1ccc2c(c1)CCOC2 | 0.103906 | 5.80 | non_toxic | 65.8 | 1 | 8,10 | 134.178 | 1.7593 | 9.23 | 2.0 |
| 1 | 9H-fluorene | 86-73-7 | c1ccc2c(c1)Cc1ccccc1-2 | 0.010460 | 5.82 | toxic | 90.0 | 1 | 7 | 166.223 | 3.2578 | 0.00 | 3.0 |
| 2 | 1,2,3,4-tetrahydronaphthalene | 119-64-2 | c1ccc2c(c1)CCCC2 | 0.020589 | 5.74 | toxic | 69.4 | 1 | 7,10 | 132.206 | 2.5654 | 0.00 | 2.0 |
| 3 | ethylbenzene | 100-41-4 | CCc1ccccc1 | 0.048107 | 5.87 | non_toxic | 65.0 | 1 | 1,2 | 106.168 | 2.2490 | 0.00 | 1.0 |
| 4 | cyclohexene | 110-83-8 | C1=CCCCC1 | 0.060688 | 5.66 | non_toxic | 96.4 | 1 | 3,6 | 82.146 | 2.1166 | 0.00 | 1.0 |
df10.head()
| Compound Name | CAS | SMILES | Solubility_mol_per_L | pKa | Toxicity | Melting Point | Reactivity | Oxidation Site | MolWt | LogP | TPSA | NumRings | NumHAcceptors | NumHDonors | NumRotatableBonds | HeavyAtomCount | FractionCSP3 | NumAromaticRings | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3,4-dihydro-1H-isochromene | 493-05-0 | c1ccc2c(c1)CCOC2 | 0.103906 | 5.80 | non_toxic | 65.8 | 1 | 8,10 | 134.178 | 1.7593 | 9.23 | 2.0 | 1.0 | 0.0 | 0.0 | 10.0 | 0.333333 | 1.0 |
| 1 | 9H-fluorene | 86-73-7 | c1ccc2c(c1)Cc1ccccc1-2 | 0.010460 | 5.82 | toxic | 90.0 | 1 | 7 | 166.223 | 3.2578 | 0.00 | 3.0 | 0.0 | 0.0 | 0.0 | 13.0 | 0.076923 | 2.0 |
| 2 | 1,2,3,4-tetrahydronaphthalene | 119-64-2 | c1ccc2c(c1)CCCC2 | 0.020589 | 5.74 | toxic | 69.4 | 1 | 7,10 | 132.206 | 2.5654 | 0.00 | 2.0 | 0.0 | 0.0 | 0.0 | 10.0 | 0.400000 | 1.0 |
| 3 | ethylbenzene | 100-41-4 | CCc1ccccc1 | 0.048107 | 5.87 | non_toxic | 65.0 | 1 | 1,2 | 106.168 | 2.2490 | 0.00 | 1.0 | 0.0 | 0.0 | 1.0 | 8.0 | 0.250000 | 1.0 |
| 4 | cyclohexene | 110-83-8 | C1=CCCCC1 | 0.060688 | 5.66 | non_toxic | 96.4 | 1 | 3,6 | 82.146 | 2.1166 | 0.00 | 1.0 | 0.0 | 0.0 | 0.0 | 6.0 | 0.666667 | 0.0 |
Now we build a 64-bit Morgan fingerprint (\(r=2\)). We keep both a small descriptor table and a high dimensional fingerprint table.
from rdkit.Chem import rdFingerprintGenerator
from rdkit import DataStructs
# --- Morgan fingerprint function (compact string) ---
def morgan_bits(smiles: str, n_bits: int = 64, radius: int = 2):
if smiles is None:
return np.nan
m = Chem.MolFromSmiles(smiles)
if m is None:
return np.nan
gen = rdFingerprintGenerator.GetMorganGenerator(radius=radius, fpSize=n_bits)
fp = gen.GetFingerprint(m)
arr = np.zeros((n_bits,), dtype=int)
DataStructs.ConvertToNumpyArray(fp, arr)
# join into one string of "0101..."
return "".join(map(str, arr))
#1024bit
df_morgan_1024 = df_raw.copy()
df_morgan_1024["Fingerprint"] = df_morgan_1024["SMILES"].apply(lambda s: morgan_bits(s, n_bits=1024, radius=2))
#64 bit
df_morgan = df_raw.copy()
df_morgan["Fingerprint"] = df_morgan["SMILES"].apply(lambda s: morgan_bits(s, n_bits=64, radius=2))
print("Rows x Cols:", df_morgan.shape)
df_morgan.head()
Rows x Cols: (575, 10)
| Compound Name | CAS | SMILES | Solubility_mol_per_L | pKa | Toxicity | Melting Point | Reactivity | Oxidation Site | Fingerprint | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3,4-dihydro-1H-isochromene | 493-05-0 | c1ccc2c(c1)CCOC2 | 0.103906 | 5.80 | non_toxic | 65.8 | 1 | 8,10 | 1000000010111000110010100000001001001000000010... |
| 1 | 9H-fluorene | 86-73-7 | c1ccc2c(c1)Cc1ccccc1-2 | 0.010460 | 5.82 | toxic | 90.0 | 1 | 7 | 1100000000000010010010101000101010001000000010... |
| 2 | 1,2,3,4-tetrahydronaphthalene | 119-64-2 | c1ccc2c(c1)CCCC2 | 0.020589 | 5.74 | toxic | 69.4 | 1 | 7,10 | 1000100000100000010010100000001000001000000010... |
| 3 | ethylbenzene | 100-41-4 | CCc1ccccc1 | 0.048107 | 5.87 | non_toxic | 65.0 | 1 | 1,2 | 1000010100000001110000100010000001001010001000... |
| 4 | cyclohexene | 110-83-8 | C1=CCCCC1 | 0.060688 | 5.66 | non_toxic | 96.4 | 1 | 3,6 | 0000101000000000010000000000001000000001100000... |
Data choices
X_small uses 4 descriptors. Good for first PCA stories.
X_fp has 64 bits. Good for t-SNE or UMAP since it is very high dimensional.
Now, let’s compare their 4D, 10D descriptors with 64D fingerprints.
import random
from rdkit.Chem import Draw
# pick indices you want
sample_indices = [1, 5, 7, 15, 20, 80, 100]
# make sure you have all descriptor sets ready
desc4 = df_raw["SMILES"].apply(calc_descriptors)
desc10 = df_raw["SMILES"].apply(calc_descriptors10)
df_all = pd.concat([df_raw, desc4, desc10.add_prefix("d10_"), df_morgan["Fingerprint"]], axis=1)
# loop through chosen indices
for idx in sample_indices:
row = df_all.iloc[idx]
mol = Chem.MolFromSmiles(row["SMILES"])
img = Draw.MolToImage(mol, size=(200, 200), legend=row["Compound Name"])
display(img)
print(f"=== {row['Compound Name']} ===")
print("SMILES:", row["SMILES"])
# 4-descriptor set
d4 = np.round([row["MolWt"], row["LogP"], row["TPSA"], row["NumRings"]], 2)
print("Descriptors [MolWt, LogP, TPSA, NumRings]:", d4)
# 10-descriptor set
d10_keys = ["MolWt","LogP","TPSA","NumRings","NumHAcceptors","NumHDonors",
"NumRotatableBonds","HeavyAtomCount","FractionCSP3","NumAromaticRings"]
d10 = np.round([row["d10_"+k] for k in d10_keys], 2)
print("10-Descriptor Vector:", d10)
print("Fingerprint:", row["Fingerprint"][:32])
print()
=== 9H-fluorene ===
SMILES: c1ccc2c(c1)Cc1ccccc1-2
Descriptors [MolWt, LogP, TPSA, NumRings]: [166.22 3.26 0. 3. ]
10-Descriptor Vector: [1.6622e+02 3.2600e+00 0.0000e+00 3.0000e+00 0.0000e+00 0.0000e+00
0.0000e+00 1.3000e+01 8.0000e-02 2.0000e+00]
Fingerprint: 11000000000000100100101010001010
=== thiolane ===
SMILES: C1CCSC1
Descriptors [MolWt, LogP, TPSA, NumRings]: [88.18 1.51 0. 1. ]
10-Descriptor Vector: [88.18 1.51 0. 1. 1. 0. 0. 5. 1. 0. ]
Fingerprint: 00001100000000010000010000000010
=== methoxymethylbenzene ===
SMILES: COCc1ccccc1
Descriptors [MolWt, LogP, TPSA, NumRings]: [122.17 1.83 9.23 1. ]
10-Descriptor Vector: [122.17 1.83 9.23 1. 1. 0. 2. 9. 0.25 1. ]
Fingerprint: 10000100010000001100001000100000
=== oct-1-ene ===
SMILES: C=CCCCCCC
Descriptors [MolWt, LogP, TPSA, NumRings]: [112.22 3.14 0. 0. ]
10-Descriptor Vector: [112.22 3.14 0. 0. 0. 0. 5. 8. 0.75 0. ]
Fingerprint: 00000000000000011100001000100001
=== N,N-diethylacetamide ===
SMILES: CCN(CC)C(C)=O
Descriptors [MolWt, LogP, TPSA, NumRings]: [115.18 0.87 20.31 0. ]
10-Descriptor Vector: [115.18 0.87 20.31 0. 1. 0. 2. 8. 0.83 0. ]
Fingerprint: 00000000001000001010000000000100
=== cyclohexane ===
SMILES: C1CCCCC1
Descriptors [MolWt, LogP, TPSA, NumRings]: [84.16 2.34 0. 1. ]
10-Descriptor Vector: [84.16 2.34 0. 1. 0. 0. 0. 6. 1. 0. ]
Fingerprint: 00101000000000000000000000000010
=== 1,4-xylene ===
SMILES: Cc1ccc(C)cc1
Descriptors [MolWt, LogP, TPSA, NumRings]: [106.17 2.3 0. 1. ]
10-Descriptor Vector: [106.17 2.3 0. 1. 0. 0. 0. 8. 0.25 1. ]
Fingerprint: 10000000000000000100001000000001
The 4-descriptor table is easy to standardize and to interpret.
The 1024-bit fingerprint captures substructure presence or absence and is useful for neighborhood maps.
With 4-desciptor, it’s easier to visualize:
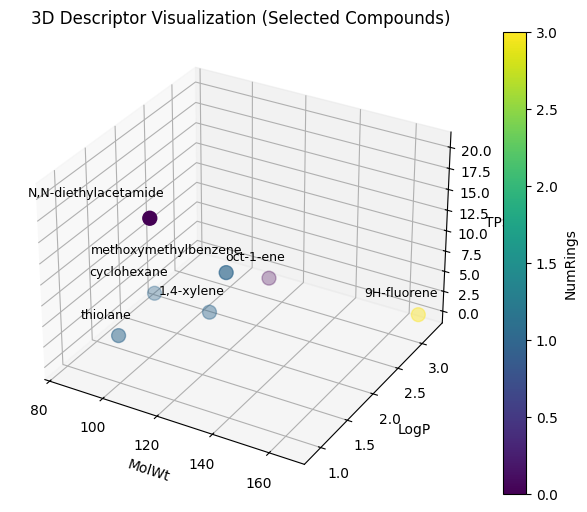
Now we can apply this to the entire C-H oxidation dataset.
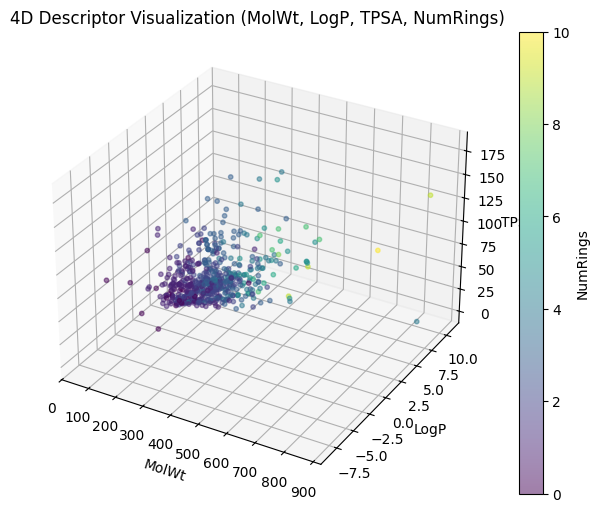
And depending which three descriptors you used for XYZ, you can have different plottings.
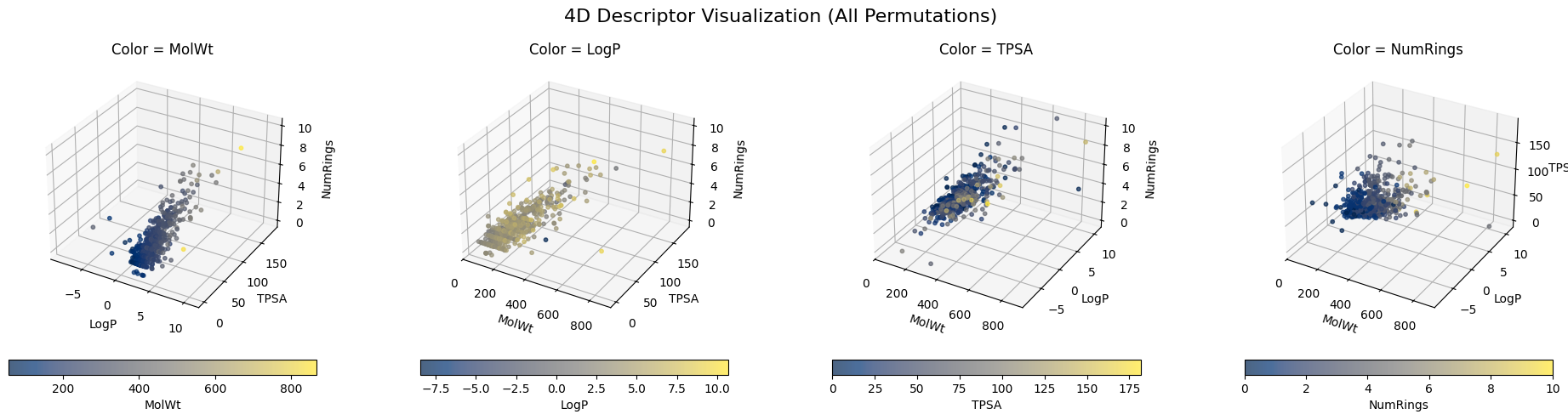
But when it comes to 10-dimensional descriptors or even something like a 1024-dimensional Morgan fingerprint, it is nearly impossible to directly visualize them. It becomes even harder to know the “neigbors” to a specific molecule.
Humans can only intuitively grasp two or three axes at once. Once you go beyond that, you can only look at pairs of dimensions at a time, and the relationships quickly become hard to characterize.
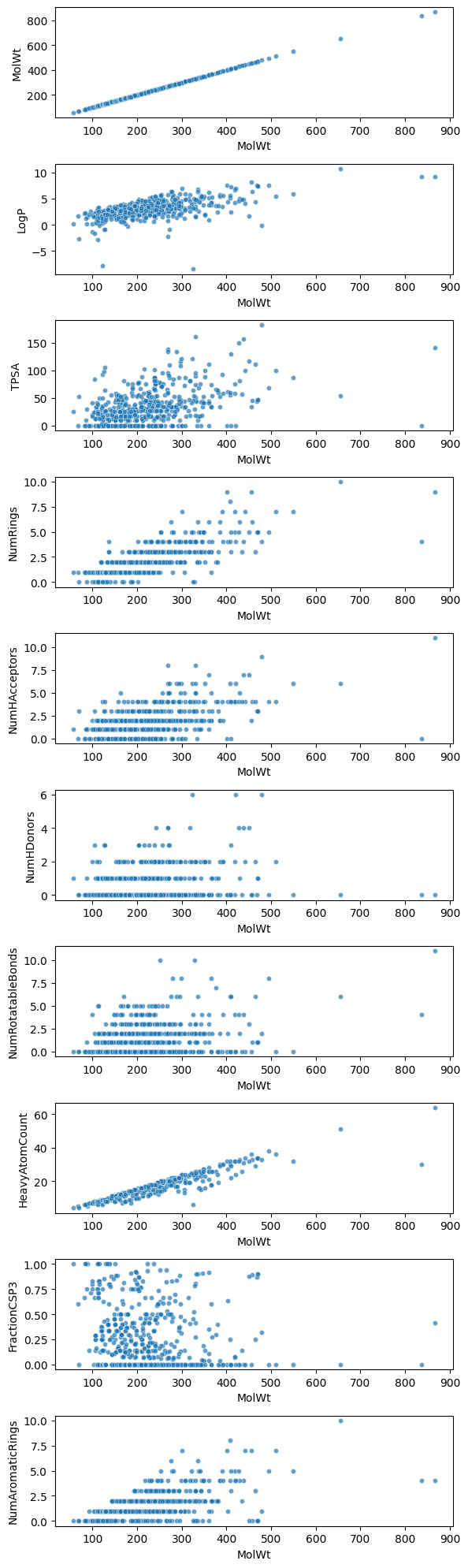
That’s why cheminformatics usually applies dimension reduction methods (PCA, t-SNE, UMAP) to compress high-dimensional data into a visualizable 2D or 3D form. We will get to these points today!
2. Supervised vs unsupervised#
In Lectures 6 to 8 we learned supervised models that map \(x \to y\) with labeled targets. Today we switch to unsupervised learning.
Dimension reduction: summarize high dimensional \(x \in \mathbb{R}^p\) to a few coordinates \(z \in \mathbb{R}^k\) with \(k \ll p\). You do not use \(y\) during the fit.
Clustering: group samples into clusters based on a similarity or distance rule. Again no labels during the fit.
We will color plots using toxicity when available. That is only to interpret the embedding or clusters. It is not used in the algorithms.
⏰ Exercise 2
State whether each task is supervised or unsupervised:
Predict melting point from descriptors.
Group molecules by scaffold similarity.
Map 1024-bit fingerprints to 2D for plotting.
Predict toxicity from descriptors.
2.1 Principal Component Analysis (PCA)#
Feature choices will drive everything downstream. You can use:
Scalar descriptors like MolWt, LogP, TPSA, ring counts.
Fingerprints like Morgan bits or MACCS keys.
Learned embeddings from a graph neural network. We will not use those here, but the same methods apply.
If two students use different features, their dimension reduction plots will look different. That is expected.
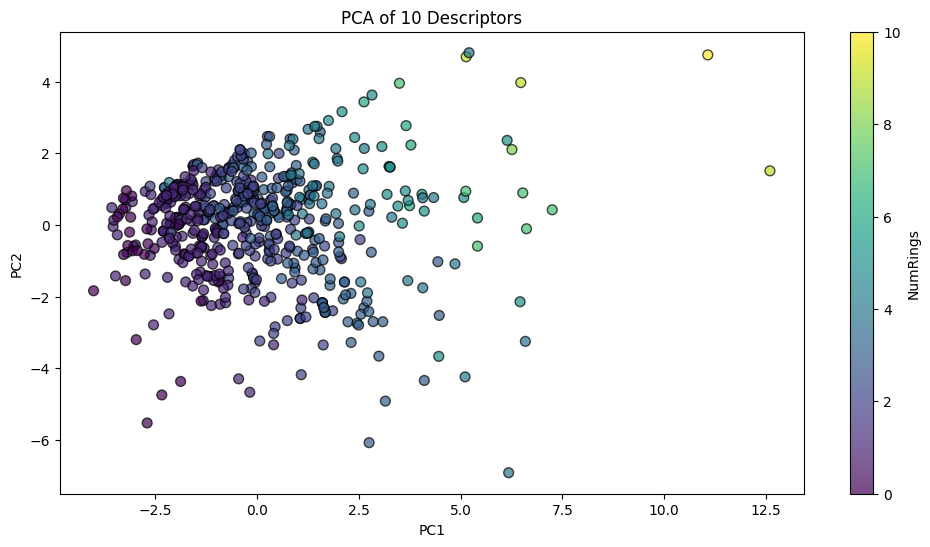
So, how do we start with dimension reduction? Let’s first try Principal Component Analysis (PCA) In the previous section, when we move from 4 descriptors (MolWt, LogP, TPSA, NumRings) to 10 descriptors or even 64-bit Morgan fingerprints, direct visualization becomes impossible. You can only plot a few dimensions at a time, and the relationships across all features become hard to interpret.
PCA addresses this challenge by finding new axes (principal components) that summarize the directions of greatest variance in the data. These axes are:
Orthogonal (uncorrelated).
Ranked by importance (PC1 explains the most variance, PC2 the second most, and so on).
Linear combinations of the original features.
Thus, PCA compresses high-dimensional molecular representations into just 2 or 3 coordinates that can be plotted.
Below is the mathematical pieces:
Start with standardized data \(\tilde X\) (mean 0, variance 1 across features).
Compute the covariance matrix: \( S = \frac{1}{n-1}\tilde X^\top \tilde X \)
Either:
Eigen-decompose \(S\) → eigenvectors are principal directions.
Or compute SVD: \( \tilde X = U \Sigma V^\top \)
The top \(k\) eigenvectors (or columns of \(V\)) form the principal directions.
The new coordinates are: \( Z = \tilde X V_k \) where \(Z\) are the principal component scores.
What this means for our chemistry dataset (df10)
df10contains 10 molecular descriptors for each compound:MolWt, LogP, TPSA, NumRings,
NumHAcceptors, NumHDonors,
NumRotatableBonds, HeavyAtomCount,
FractionCSP3, NumAromaticRings.
These form a data matrix \(X\) of shape (n molecules × 10 features).
PCA standardizes each column (feature) so they are comparable on the same scale.
For example, MolWt ranges in the hundreds, but FractionCSP3 is between 0 and 1. Standardization makes both contribute fairly.Then PCA computes principal directions as linear combinations of these 10 descriptors.
The result is a new score matrix \(Z\) of shape (n molecules × k components), usually with \(k=2\) or \(3\) for visualization.
This means each molecule is now represented by just 2–3 numbers (PC1, PC2, PC3) instead of 10 descriptors.The same approach works for fingerprints: instead of 10 columns, you may have 64, 1024, or 2048. PCA reduces that down to 2–3 interpretable axes.
from sklearn.preprocessing import StandardScaler
from sklearn.decomposition import PCA
import matplotlib.pyplot as plt
from mpl_toolkits.mplot3d import Axes3D
import numpy as np
# ---- prepare matrices ----
X_desc = df10[["MolWt","LogP","TPSA","NumRings",
"NumHAcceptors","NumHDonors","NumRotatableBonds",
"HeavyAtomCount","FractionCSP3","NumAromaticRings"]].values
X_morgan = np.array([[int(ch) for ch in fp] for fp in df_morgan["Fingerprint"]]) # 64 bits
# ---- standardize ----
X_desc_std = StandardScaler().fit_transform(X_desc)
X_morgan_std = StandardScaler().fit_transform(X_morgan)
# ---- PCA fit ----
pca_desc = PCA().fit(X_desc_std)
pca_morgan = PCA().fit(X_morgan_std)
# ---- scree plots ----
fig, axes = plt.subplots(1, 2, figsize=(12,4))
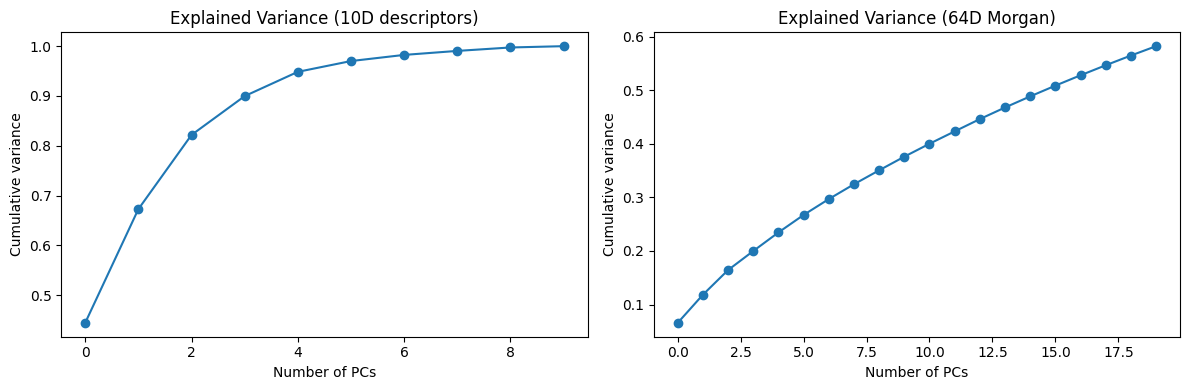
axes[0].plot(np.cumsum(pca_desc.explained_variance_ratio_[:10]), marker="o")
axes[0].set_title("Explained Variance (10D descriptors)")
axes[0].set_xlabel("Number of PCs")
axes[0].set_ylabel("Cumulative variance")
axes[1].plot(np.cumsum(pca_morgan.explained_variance_ratio_[:20]), marker="o")
axes[1].set_title("Explained Variance (64D Morgan)")
axes[1].set_xlabel("Number of PCs")
axes[1].set_ylabel("Cumulative variance")
plt.tight_layout()
plt.show()
# ---- 2D PCA projections ----
desc_2d = pca_desc.transform(X_desc_std)[:, :2]
morgan_2d = pca_morgan.transform(X_morgan_std)[:, :2]
fig, axes = plt.subplots(1, 2, figsize=(12,5))
axes[0].scatter(desc_2d[:,0], desc_2d[:,1], c="blue", alpha=0.7)
axes[0].set_title("PCA 2D projection (10 descriptors)")
axes[0].set_xlabel("PC1")
axes[0].set_ylabel("PC2")
axes[1].scatter(morgan_2d[:,0], morgan_2d[:,1], c="green", alpha=0.7)
axes[1].set_title("PCA 2D projection (64-bit Morgan)")
axes[1].set_xlabel("PC1")
axes[1].set_ylabel("PC2")
plt.tight_layout()
plt.show()
# ---- 3D PCA projections ----
desc_3d = pca_desc.transform(X_desc_std)[:, :3]
morgan_3d = pca_morgan.transform(X_morgan_std)[:, :3]
fig = plt.figure(figsize=(12,5))
ax1 = fig.add_subplot(121, projection="3d")
ax1.scatter(desc_3d[:,0], desc_3d[:,1], desc_3d[:,2],
c="blue", alpha=0.7, s=50)
ax1.set_title("PCA 3D projection (10 descriptors)")
ax1.set_xlabel("PC1"); ax1.set_ylabel("PC2"); ax1.set_zlabel("PC3")
ax2 = fig.add_subplot(122, projection="3d")
ax2.scatter(morgan_3d[:,0], morgan_3d[:,1], morgan_3d[:,2],
c="green", alpha=0.7, s=50)
ax2.set_title("PCA 3D projection (64-bit Morgan)")
ax2.set_xlabel("PC1"); ax2.set_ylabel("PC2"); ax2.set_zlabel("PC3")
plt.tight_layout()
plt.show()
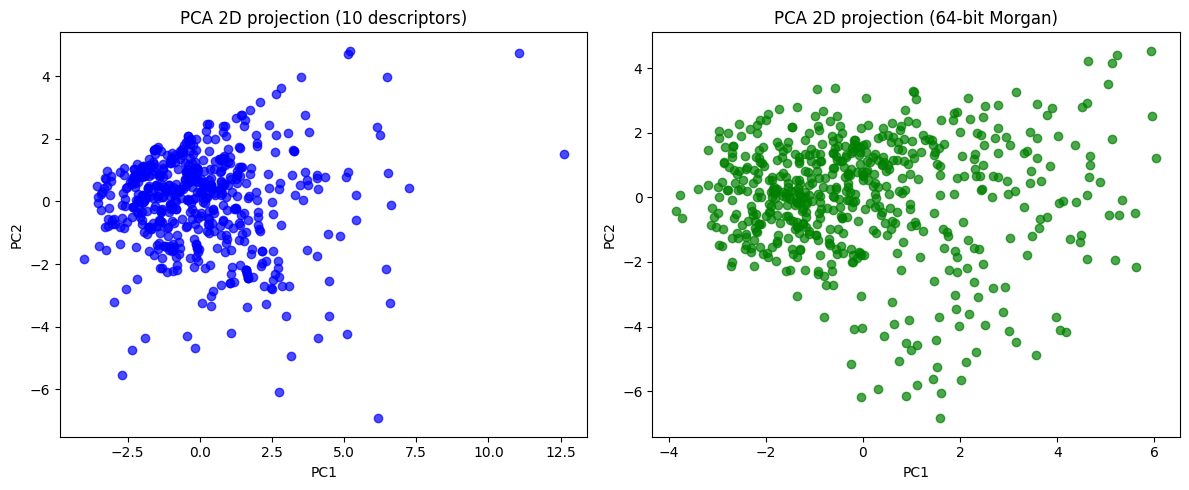
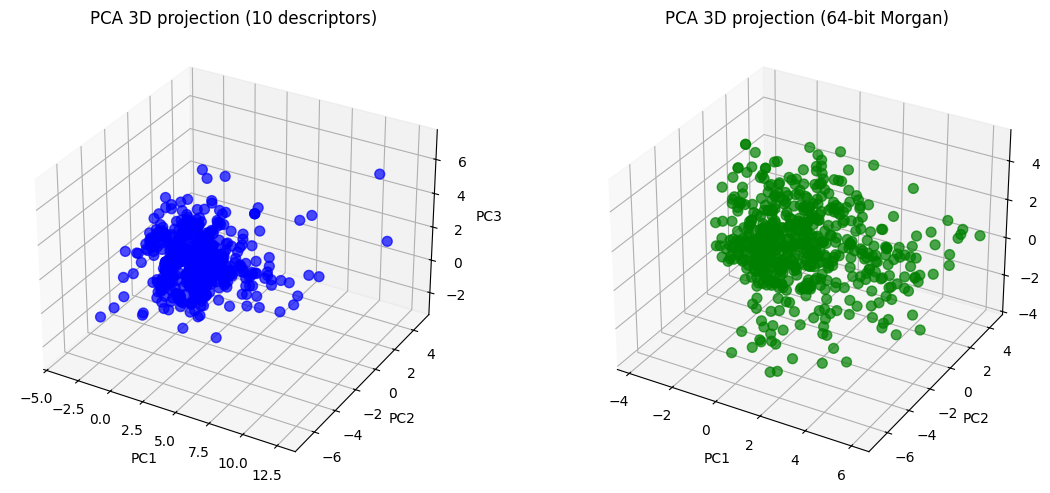
Now you can see, the 10D vector is converted to 2D or 3D vector, which become much easier to plot.
desc_2d
array([[-1.85863457, 0.10406515],
[-0.8662983 , 1.26269482],
[-2.08798314, 0.77811 ],
...,
[ 0.08373577, 0.58690452],
[ 3.19411974, 0.85437761],
[ 2.00081897, 0.04085639]], shape=(575, 2))
desc_3d
array([[-1.85863457, 0.10406515, -0.76130014],
[-0.8662983 , 1.26269482, -1.55622595],
[-2.08798314, 0.77811 , -0.75279025],
...,
[ 0.08373577, 0.58690452, 0.27978755],
[ 3.19411974, 0.85437761, 0.07107396],
[ 2.00081897, 0.04085639, 0.17583671]], shape=(575, 3))
In essence, PCA gives us coordinates for each molecule in a reduced 2D space.
To make this more concrete, we can select specific molecules and compare them:
A manual pair that we choose (indices 0 and 5).
The closest pair in PCA space (most similar according to the descriptors).
The farthest pair in PCA space (most dissimilar).
We plot all molecules in grey, highlight the pairs with different colors, and draw a line connecting each pair.
For the selected molecules we also show their structure, compound name, and PCA coordinates.
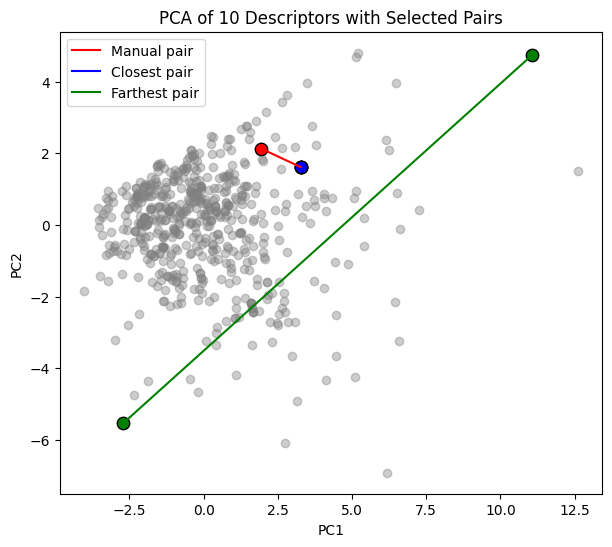
=== Manual pair ===
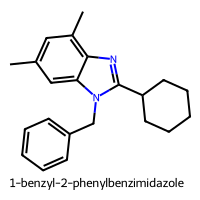
1-benzyl-2-phenylbenzimidazole (index 225)
PC1 = 1.936, PC2 = 2.132
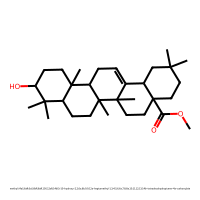
methyl (4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylate (index 35)
PC1 = 3.267, PC2 = 1.618
=== Closest pair ===

methyl (4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylate (index 35)
PC1 = 3.267, PC2 = 1.618
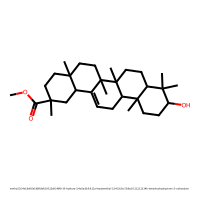
methyl (2S,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bR)-10-hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-2-carboxylate (index 64)
PC1 = 3.267, PC2 = 1.618
=== Farthest pair ===
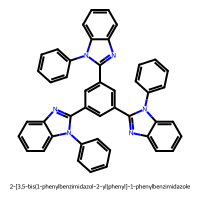
2-[3,5-bis(1-phenylbenzimidazol-2-yl)phenyl]-1-phenylbenzimidazole (index 338)
PC1 = 11.072, PC2 = 4.750
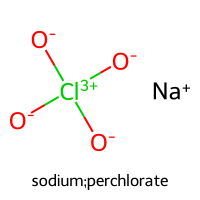
sodium;perchlorate (index 462)
PC1 = -2.699, PC2 = -5.531
We can also make this plot to be interactive:
2.2 Data leakage in unsupervised settings#
Even in unsupervised workflows, leakage can happen.
A common mistake is to fit the scaler or PCA on the full dataset before splitting.
This means the test set has already influenced the scaling or component directions, which is a subtle form of information leak.
Safer pattern:
Split the dataset into train and test.
Fit the scaler only on the training subset.
Transform both train and test using the fitted scaler.
Fit PCA (or clustering) on the training data only, then apply the transform to the test set.
This way the test set truly remains unseen during model fitting.
Train shape: (402, 2)
Test shape: (173, 2)
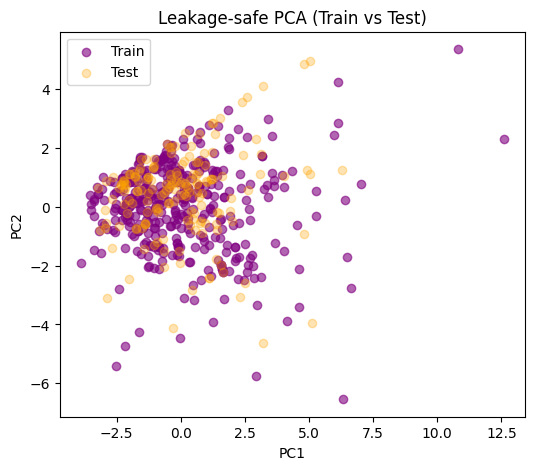
If the test set overlaps reasonably with the distribution of the train set, that’s a sign your train-derived scaling and PCA directions generalize.
If the test points lie far outside, that could mean:
Train and test distributions are very different.
Or PCA didn’t capture the structure of test molecules well. Note:
In pure visualization use cases, the risk is smaller since we are not optimizing a predictive model.
For cluster analysis that will be compared to labels afterward, keep the split discipline.
3. Standardization and distance#
Unsupervised methods depend on a distance or similarity rule. The choice of distance and the way you scale features can completely change neighborhoods, clusters, and embeddings. This section covers why we standardize, which distance to use for scalar descriptors vs fingerprints, and how to compute and visualize distance in a leakage safe way.
3.1 Why standardize#
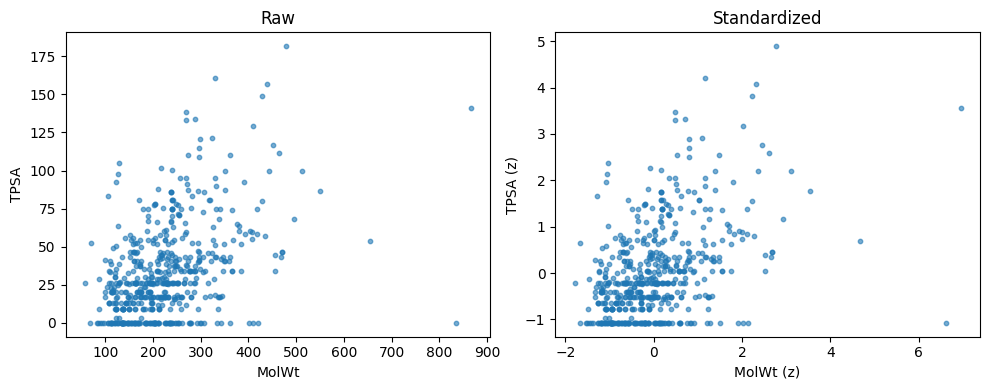
Descriptor columns live on different scales. MolWt can be in the hundreds. FractionCSP3 is in [0, 1]. If you compute Euclidean distance on the raw matrix, large scale columns dominate. Standardization fixes this by centering and scaling each column so that all features contribute fairly.
Common scalers:
StandardScaler: zero mean, unit variance. Good default.
MinMaxScaler: rescales to [0, 1]. Useful when you want bounded ranges.
RobustScaler: uses medians and IQR. Useful with outliers.
Below is an example for StandardScaler()
from sklearn.preprocessing import StandardScaler
# pick two columns
cols2 = ["MolWt", "TPSA"]
X2 = df10[cols2].to_numpy()
# standardize both columns
scaler = StandardScaler().fit(X2)
X2_std = scaler.transform(X2)
# show means and std before vs after
print("Raw means:", np.round(X2.mean(axis=0), 3))
print("Raw stds :", np.round(X2.std(axis=0, ddof=0), 3))
print("Std means:", np.round(X2_std.mean(axis=0), 3))
print("Std stds :", np.round(X2_std.std(axis=0, ddof=0), 3))
# quick scatter before vs after
fig, ax = plt.subplots(1, 2, figsize=(10,4))
ax[0].scatter(X2[:,0], X2[:,1], s=10, alpha=0.6)
ax[0].set_xlabel("MolWt"); ax[0].set_ylabel("TPSA"); ax[0].set_title("Raw")
ax[1].scatter(X2_std[:,0], X2_std[:,1], s=10, alpha=0.6)
ax[1].set_xlabel("MolWt (z)"); ax[1].set_ylabel("TPSA (z)"); ax[1].set_title("Standardized")
plt.tight_layout(); plt.show()
Raw means: [223.213 32.74 ]
Raw stds : [92.66 30.417]
Std means: [ 0. -0.]
Std stds : [1. 1.]
3.2 Which distance to use#
Scalar descriptors (10D):
Euclidean on standardized data is a good default.
Cosine focuses on direction rather than magnitude. Try it when overall size varies a lot.
Fingerprints (64D or 1024D binary bits):
Tanimoto similarity is the chem-informatics standard. Tanimoto distance = 1 − Tanimoto similarity.
You can also compute cosine, but Tanimoto aligns with bitset overlap and is more common for substructure style features.
Below are equations:
Euclidean distance between rows \(x_i, x_j \in \mathbb{R}^d\)
\( d_{\text{Euc}}(i,j)=\left\|x_i-x_j\right\|_2=\sqrt{\sum_{k=1}^{d}\left(x_{ik}-x_{jk}\right)^2} \)Cosine distance (1 minus cosine similarity)
\( d_{\text{Cos}}(i,j)=1-\frac{x_i^\top x_j}{\lVert x_i\rVert_2\,\lVert x_j\rVert_2} \)Tanimoto distance for bit vectors \(b_i,b_j\in\{0,1\}^m\) with on-bit sets \(A,B\)
\( s_{\text{Tan}}(i,j)=\frac{|A\cap B|}{|A|+|B|-|A\cap B|},\qquad d_{\text{Tan}}(i,j)=1-s_{\text{Tan}}(i,j) \)
Below we show Euclidean vs Cosine on two standardized columns
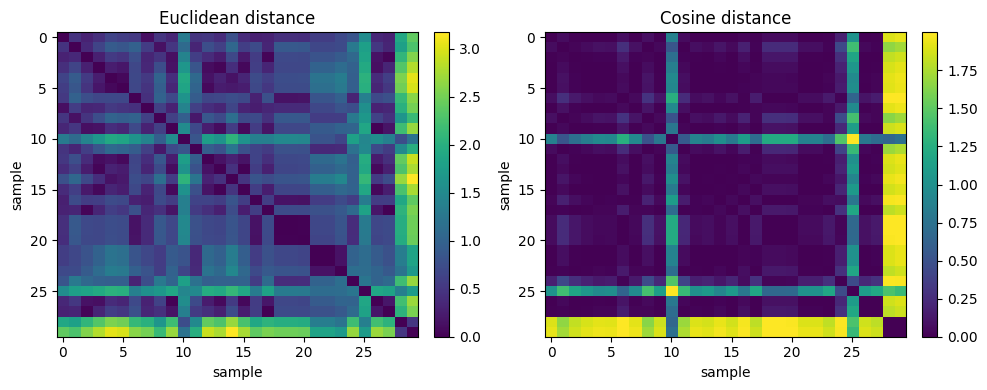
Euclidean Nearest Neighbor (rows) for sample #10: [23, 28, 21, 22, 11]
Cosine Nearest Neighbor (rows) for sample #10: [8, 1, 11, 28, 27]
Note
Idea is that, the same query point can have different nearest neighbors under Euclidean vs Cosine because one metric cares about absolute offsets while the other cares about direction.
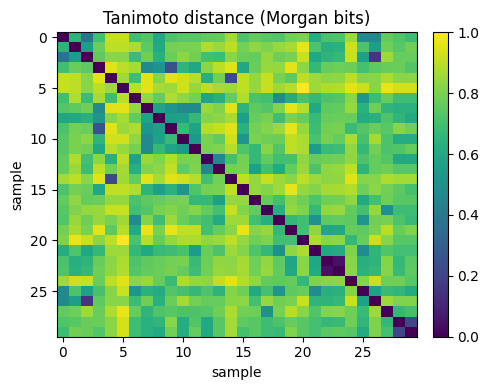
Tanimoto NN (rows) for query 10: [7, 15, 11, 8, 28]
Note
Idea is that, small Tanimoto distance means strong bit overlap, which often reflects shared substructures. This is why Tanimoto is standard for fingerprint similarity.
Below we add a simple 2D plot to show how nearest neighbors can differ under different metrics in the same fingerprint space.
We compute a 2D embedding once (PCA on the 0/1 bit matrix) and then draw neighbor connections for all three kinds of distance calculation methods.
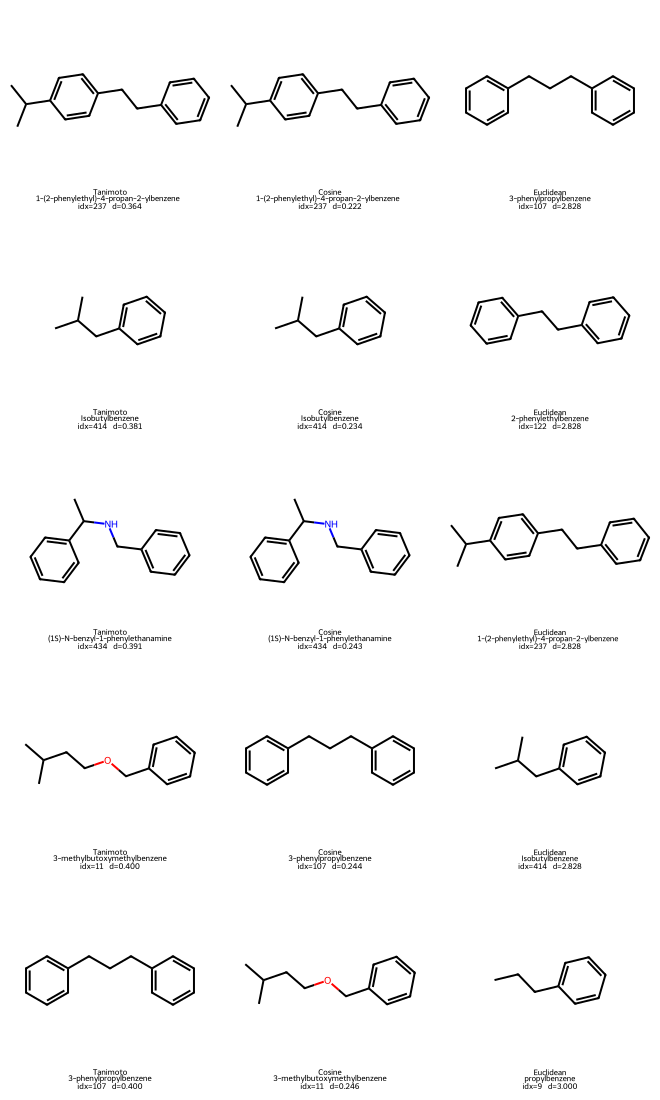
Tanimoto NN for query 196 -> [237, 414, 434, 11, 107]
Cosine NN for query 196 -> [237, 414, 434, 107, 11]
Euclidean NN for query 196 -> [107, 122, 237, 414, 9]
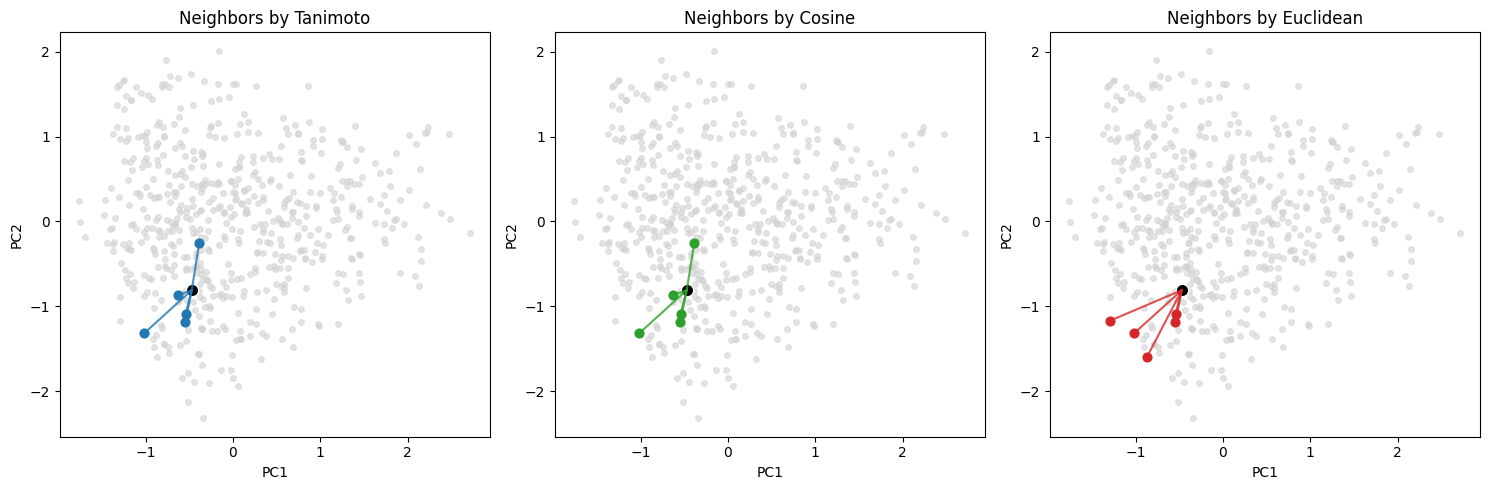
We can plot both the query molecule and the found neighbors to take a look:
Query #196: 3-methylbutylbenzene
4. Nonlinear embeddings: t-SNE and UMAP#
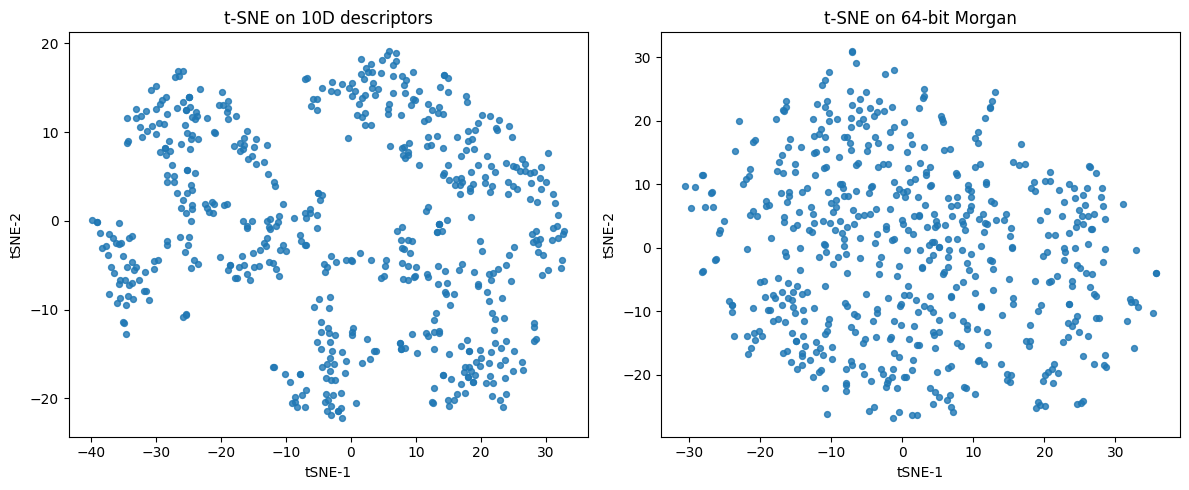
Linear PCA gives a fast global summary. For curved manifolds or cluster-heavy data, t-SNE and UMAP often reveal structure that PCA compresses. Both start from a notion of neighborhood in the original feature space and then optimize a 2D or 3D layout that tries to keep neighbors close.
We will use two feature sets:
10D scalar descriptors from
df10[desc_cols]64-bit Morgan fingerprints from
df_morgan["Fingerprint"]
4.1 t-SNE#
Idea: Build probabilities that say which points are neighbors in high-dim, then find a low-dim map whose neighbor probabilities match.
Math:
Convert distances to conditional probabilities with a Gaussian kernel per point: \( p_{j\mid i}=\frac{\exp\!\left(-\frac{\lVert x_i-x_j\rVert^2}{2\sigma_i^2}\right)}{\sum_{k\ne i}\exp\!\left(-\frac{\lVert x_i-x_k\rVert^2}{2\sigma_i^2}\right)},\quad p_{i\mid i}=0 \) \(\sigma_i\) is chosen so that the perplexity matches a user target (roughly the effective number of neighbors).
Symmetrize: \( P_{ij}=\frac{p_{j\mid i}+p_{i\mid j}}{2n} \)
In 2D, use a heavy-tailed kernel to define \( q_{ij}=\frac{\left(1+\lVert y_i-y_j\rVert^2\right)^{-1}}{\sum_{k\ne \ell}\left(1+\lVert y_k-y_\ell\rVert^2\right)^{-1}},\quad q_{ii}=0 \)
Optimize by minimizing the Kullback–Leibler divergence \( \operatorname{KL}(P\parallel Q)=\sum_{i\ne j}P_{ij}\log\frac{P_{ij}}{Q_{ij}}. \)/
Practical tips:
Choose
perplexityabout 5 to 50. For ~500 molecules, start at 30.Standardize descriptor matrices. Fingerprints are binary. You can feed them as 0/1, or first reduce to 50 PCs for speed.
from sklearn.manifold import TSNE
# ----- features -----
desc_cols = ["MolWt","LogP","TPSA","NumRings",
"NumHAcceptors","NumHDonors","NumRotatableBonds",
"HeavyAtomCount","FractionCSP3","NumAromaticRings"]
# 10D descriptors: standardize
X_desc = df10[desc_cols].to_numpy()
X_desc_std = StandardScaler().fit_transform(X_desc)
# 64-bit Morgan: turn "0101..." into 0/1 matrix
X_fp = np.array([[int(ch) for ch in s] for s in df_morgan["Fingerprint"]], dtype=float)
# optional: speed-up via PCA(50) before t-SNE on fingerprints
#X_fp_50 = PCA(n_components=min(50, X_fp.shape[1], X_fp.shape[0]-1), random_state=0).fit_transform(X_fp)
# ----- t-SNE fits -----
tsne_desc = TSNE(n_components=2, perplexity=30, learning_rate="auto",
init="pca", random_state=0)
Y_desc = tsne_desc.fit_transform(X_desc_std)
tsne_fp = TSNE(n_components=2, perplexity=30, learning_rate="auto",
init="pca", random_state=0)
Y_fp = tsne_fp.fit_transform(X_fp)
# ----- plots -----
fig, ax = plt.subplots(1, 2, figsize=(12,5))
ax[0].scatter(Y_desc[:,0], Y_desc[:,1], s=18, alpha=0.8)
ax[0].set_title("t-SNE on 10D descriptors")
ax[0].set_xlabel("tSNE-1"); ax[0].set_ylabel("tSNE-2")
ax[1].scatter(Y_fp[:,0], Y_fp[:,1], s=18, alpha=0.8)
ax[1].set_title("t-SNE on 64-bit Morgan")
ax[1].set_xlabel("tSNE-1"); ax[1].set_ylabel("tSNE-2")
plt.tight_layout(); plt.show()
4.2 UMAP#
Idea: Build a weighted kNN graph in the original space, interpret it as a fuzzy set of edges, then find low dimensional coordinates that preserve this fuzzy structure.
Math:
For each point (i), choose a local connectivity scale so that exactly (k) neighbors have significant membership. Convert distances to directed fuzzy memberships \(\mu_{i\to j}\in[0,1]\).
Symmetrize with fuzzy union \( \mu_{ij} = \mu_{i\to j} + \mu_{j\to i} - \mu_{i\to j}\mu_{j\to i} \)
In low dimension, define a differentiable edge likelihood controlled by spread and min_dist. Optimize a cross entropy between the high dimensional and low dimensional fuzzy graphs with negative sampling.
Practice:
n_neighborssets the balance of local vs global. Small values focus on fine detail, larger values keep more global structure. Start at 15 to 50.min_distcontrols cluster tightness. Smaller values allow tighter blobs, larger values spread points.UMAP supports
.transform, so you can fit on train then place test without leakage. For fingerprints,metric="jaccard"matches Tanimoto on 0 or 1 bits.
Note
We will skip the code here, please them run in Colab.
So how these compare in chemistry?
In terms of global shape:
PCA preserves coarse directions of variance and is easy to interpret through loadings. -UMAP can keep some global relations if
n_neighborsis set higher. -t-SNE focuses on local neighborhoods and often distorts large scale distances.
For clusters:
t-SNE and UMAP usually separate clusters more clearly than PCA.
UMAP tends to preserve relative distances between clusters better than t-SNE.
Workflow tips.
Decide the feature family. For scalar descriptors, standardize first. For fingerprints, keep bits and pick a metric that matches chemistry.
If you plan any evaluation, split first. Fit scalers and embedding models on train only. Transform test afterward.
Read maps with labels only for interpretation. Do not feed labels during fitting in unsupervised sections.
Summary: PCA finds orthogonal directions of variance. t-SNE preserves neighbor probabilities and is great for tight clusters. UMAP preserves a fuzzy kNN graph and supports transforming new points. On molecules, try both t-SNE and UMAP on descriptors and fingerprints, then read the plots with domain knowledge rather than expecting one single correct picture.
5. Glossary#
- dimension reduction#
Map \(p\)-dimensional features to a few coordinates that keep the most structure for your task.
- PCA#
Linear method that finds orthogonal directions of maximum variance.
- loading#
The weight of each original feature for a principal component.
- scree plot#
Bar or line plot of explained variance ratio per PC.
- t-SNE#
Nonlinear embedding that preserves local neighbor relations. Uses a KL divergence objective.
- UMAP#
Graph-based embedding that models fuzzy set cross entropy on a kNN graph.
6. In-class activity#
Scenario: You are screening 20 candidate bio-inhibitors from three chemotypes. Your goal is to build quick descriptor and fingerprint views, create 2D maps, and compare nearest neighbors under different distances before moving to assays.
# Fixed 20 compounds with groups (3 clusters)
mol_records = [
# Group A - hydrophobic aromatics
("Toluene", "Cc1ccccc1", "A_hydrophobic_aromatics"),
("Ethylbenzene", "CCc1ccccc1", "A_hydrophobic_aromatics"),
("Propylbenzene", "CCCc1ccccc1", "A_hydrophobic_aromatics"),
("p-Xylene", "Cc1ccc(cc1)C", "A_hydrophobic_aromatics"),
("Cumene", "CC(C)c1ccccc1", "A_hydrophobic_aromatics"),
("Mesitylene", "Cc1cc(C)cc(C)c1", "A_hydrophobic_aromatics"),
("Isobutylbenzene", "CC(C)Cc1ccccc1", "A_hydrophobic_aromatics"),
# Group B - polar alcohols and acids
("Acetic acid", "CC(=O)O", "B_polar_alc_acid"),
("Propionic acid", "CCC(=O)O", "B_polar_alc_acid"),
("Butanoic acid", "CCCC(=O)O", "B_polar_alc_acid"),
("Ethanol", "CCO", "B_polar_alc_acid"),
("1-Propanol", "CCCO", "B_polar_alc_acid"),
("1-Butanol", "CCCCO", "B_polar_alc_acid"),
("Ethylene glycol", "OCCO", "B_polar_alc_acid"),
# Group C - nitrogen hetero and amines
("Pyridine", "n1ccccc1", "C_N_hetero_amines"),
("Aniline", "Nc1ccccc1", "C_N_hetero_amines"),
("Dimethylaniline", "CN(C)c1ccccc1", "C_N_hetero_amines"),
("Imidazole", "c1ncc[nH]1", "C_N_hetero_amines"),
("Morpholine", "O1CCNCC1", "C_N_hetero_amines"),
("Piperidine", "N1CCCCC1", "C_N_hetero_amines"),
]
import pandas as pd
df_20 = pd.DataFrame(mol_records, columns=["Compound Name","SMILES","Group"])
df_20
| Compound Name | SMILES | Group | |
|---|---|---|---|
| 0 | Toluene | Cc1ccccc1 | A_hydrophobic_aromatics |
| 1 | Ethylbenzene | CCc1ccccc1 | A_hydrophobic_aromatics |
| 2 | Propylbenzene | CCCc1ccccc1 | A_hydrophobic_aromatics |
| 3 | p-Xylene | Cc1ccc(cc1)C | A_hydrophobic_aromatics |
| 4 | Cumene | CC(C)c1ccccc1 | A_hydrophobic_aromatics |
| 5 | Mesitylene | Cc1cc(C)cc(C)c1 | A_hydrophobic_aromatics |
| 6 | Isobutylbenzene | CC(C)Cc1ccccc1 | A_hydrophobic_aromatics |
| 7 | Acetic acid | CC(=O)O | B_polar_alc_acid |
| 8 | Propionic acid | CCC(=O)O | B_polar_alc_acid |
| 9 | Butanoic acid | CCCC(=O)O | B_polar_alc_acid |
| 10 | Ethanol | CCO | B_polar_alc_acid |
| 11 | 1-Propanol | CCCO | B_polar_alc_acid |
| 12 | 1-Butanol | CCCCO | B_polar_alc_acid |
| 13 | Ethylene glycol | OCCO | B_polar_alc_acid |
| 14 | Pyridine | n1ccccc1 | C_N_hetero_amines |
| 15 | Aniline | Nc1ccccc1 | C_N_hetero_amines |
| 16 | Dimethylaniline | CN(C)c1ccccc1 | C_N_hetero_amines |
| 17 | Imidazole | c1ncc[nH]1 | C_N_hetero_amines |
| 18 | Morpholine | O1CCNCC1 | C_N_hetero_amines |
| 19 | Piperidine | N1CCCCC1 | C_N_hetero_amines |
Q1. Build feature sets and print compact summaries#
Using
calc_descriptors10we defined earlier today, create a 20×10 descriptor tabledf_fp_20for these molecules.Using
morgan_bitswithn_bits=64andradius=2, create a 64-bit fingerprint string for each molecule.Show the first 24 bits of the fingerprint for all 20 molecules.
#TO DO
Q2. PCA on 10 descriptors#
Build a feature matrix
X_descfrom the 10 descriptor columns, then standardize withStandardScaler(), call itX_desc_std.Fit
PCA()on standardized data and project to 2D.Create a scatter. Optional: add text labels for each molecule.
#TO DO
Q3. t-SNE on 10 descriptors#
Use the same standardized 10D matrix
X_desc_std.Fit t-SNE with
n_components=2,perplexity=5,init="pca",learning_rate="auto",n_iter=1000,random_state=0.Make a scatter with text labels
Change
perplexityto2,10, and15, see how they are different from each other.
#TO DO
Q4. PCA and t-SNE on Morgan-64 fingerprints#
Convert each 64-bit fingerprint string to a row of 0 or 1 to form a 20×64 matrix ·X_fp·.
Run PCA to 2D and plot with labels and t-SNE to 2D on
X_fpwith the same t-SNE settings as Q3, and plot with labels.Compare with Q3
#TO DO
Q5. Nearest 5 neighbors - descriptors vs fingerprints#
Pick a query molecule index
qbetween0and19Compute a 20×20 Euclidean distance matrix on
X_desc_stdand list the 5 closest neighbors ofq(exclude itself).Compute a 20×20 Tanimoto distance matrix for the fingerprints and list the 5 closest neighbors of
q.Compare the two neighbor lists. Which list better respects group membership for this query?
#TO DO
7. Solution#
Q1#
# Q1 - Solution
# 10-descriptor table
desc10_20 = df_20["SMILES"].apply(calc_descriptors10)
df10_20 = pd.concat([df_20[["Compound Name","SMILES","Group"]], desc10_20], axis=1)
# 64-bit Morgan fingerprints
df_fp_20 = df_20.copy()
df_fp_20["Fingerprint"] = df_fp_20["SMILES"].apply(lambda s: morgan_bits(s, n_bits=64, radius=2))
print("10-descriptor table (head):")
display(df10_20.head().round(3))
print("\nFingerprint preview (first 24 bits):")
fp_preview = df_fp_20[["Compound Name","Group","Fingerprint"]].copy()
fp_preview["Fingerprint"] = fp_preview["Fingerprint"].str.slice(0, 24) + "..."
display(fp_preview)
print("\nDescriptor stats:")
display(df10_20.drop(columns=["Compound Name","SMILES","Group"]).agg(["mean","std"]).round(3))
10-descriptor table (head):
| Compound Name | SMILES | Group | MolWt | LogP | TPSA | NumRings | NumHAcceptors | NumHDonors | NumRotatableBonds | HeavyAtomCount | FractionCSP3 | NumAromaticRings | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Toluene | Cc1ccccc1 | A_hydrophobic_aromatics | 92.141 | 1.995 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 7.0 | 0.143 | 1.0 |
| 1 | Ethylbenzene | CCc1ccccc1 | A_hydrophobic_aromatics | 106.168 | 2.249 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 8.0 | 0.250 | 1.0 |
| 2 | Propylbenzene | CCCc1ccccc1 | A_hydrophobic_aromatics | 120.195 | 2.639 | 0.0 | 1.0 | 0.0 | 0.0 | 2.0 | 9.0 | 0.333 | 1.0 |
| 3 | p-Xylene | Cc1ccc(cc1)C | A_hydrophobic_aromatics | 106.168 | 2.303 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 8.0 | 0.250 | 1.0 |
| 4 | Cumene | CC(C)c1ccccc1 | A_hydrophobic_aromatics | 120.195 | 2.810 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 9.0 | 0.333 | 1.0 |
Fingerprint preview (first 24 bits):
| Compound Name | Group | Fingerprint | |
|---|---|---|---|
| 0 | Toluene | A_hydrophobic_aromatics | 100001000000000001000010... |
| 1 | Ethylbenzene | A_hydrophobic_aromatics | 100001010000000111000010... |
| 2 | Propylbenzene | A_hydrophobic_aromatics | 100001000000000111000110... |
| 3 | p-Xylene | A_hydrophobic_aromatics | 100000000000000001000010... |
| 4 | Cumene | A_hydrophobic_aromatics | 110001000000100001000110... |
| 5 | Mesitylene | A_hydrophobic_aromatics | 000000000000000001000000... |
| 6 | Isobutylbenzene | A_hydrophobic_aromatics | 110001000000000111000010... |
| 7 | Acetic acid | B_polar_alc_acid | 000001000010000000000000... |
| 8 | Propionic acid | B_polar_alc_acid | 001001000110000010000000... |
| 9 | Butanoic acid | B_polar_alc_acid | 000001010110010010000000... |
| 10 | Ethanol | B_polar_alc_acid | 001000000000000010000000... |
| 11 | 1-Propanol | B_polar_alc_acid | 000000000000000010000000... |
| 12 | 1-Butanol | B_polar_alc_acid | 000000000000000110000000... |
| 13 | Ethylene glycol | B_polar_alc_acid | 000000000000000010000000... |
| 14 | Pyridine | C_N_hetero_amines | 100101000010000001000000... |
| 15 | Aniline | C_N_hetero_amines | 100001000000100001010010... |
| 16 | Dimethylaniline | C_N_hetero_amines | 100001001001000001000010... |
| 17 | Imidazole | C_N_hetero_amines | 001100000110000001000000... |
| 18 | Morpholine | C_N_hetero_amines | 000100000001000010000000... |
| 19 | Piperidine | C_N_hetero_amines | 001011000000000000000000... |
Descriptor stats:
| MolWt | LogP | TPSA | NumRings | NumHAcceptors | NumHDonors | NumRotatableBonds | HeavyAtomCount | FractionCSP3 | NumAromaticRings | |
|---|---|---|---|---|---|---|---|---|---|---|
| mean | 89.882 | 1.198 | 15.858 | 0.650 | 0.750 | 0.600 | 0.700 | 6.500 | 0.51 | 0.55 |
| std | 24.965 | 1.152 | 15.012 | 0.489 | 0.639 | 0.598 | 0.801 | 2.065 | 0.38 | 0.51 |
Q2#
# Q2 - Solution
import numpy as np
import matplotlib.pyplot as plt
from sklearn.preprocessing import StandardScaler
from sklearn.decomposition import PCA
desc_cols = ["MolWt","LogP","TPSA","NumRings",
"NumHAcceptors","NumHDonors","NumRotatableBonds",
"HeavyAtomCount","FractionCSP3","NumAromaticRings"]
X_desc = df10_20[desc_cols].to_numpy()
X_desc_std = StandardScaler().fit_transform(X_desc)
pca_desc_full = PCA().fit(X_desc_std)
Z_desc = pca_desc_full.transform(X_desc_std)[:, :2]
# color map by group
group_to_color = {"A_hydrophobic_aromatics":"tab:orange",
"B_polar_alc_acid":"tab:blue",
"C_N_hetero_amines":"tab:green"}
colors = df10_20["Group"].map(group_to_color).to_numpy()
# explained variance
plt.figure(figsize=(4,3))
plt.plot(np.cumsum(pca_desc_full.explained_variance_ratio_), marker="o")
plt.xlabel("Number of PCs"); plt.ylabel("Cumulative EVR"); plt.tight_layout(); plt.show()
# 2D scatter with labels
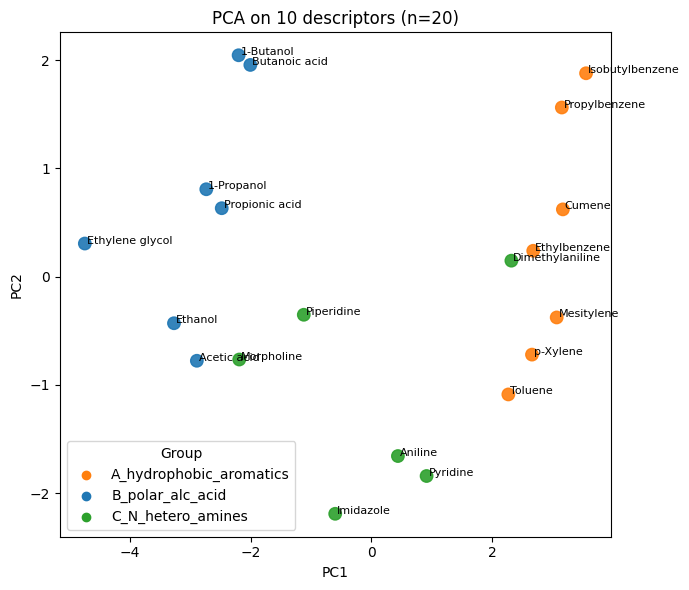
plt.figure(figsize=(7,6))
plt.scatter(Z_desc[:,0], Z_desc[:,1], s=80, c=colors, alpha=0.9)
for i, name in enumerate(df10_20["Compound Name"]):
plt.text(Z_desc[i,0]+0.03, Z_desc[i,1], name, fontsize=8)
plt.xlabel("PC1"); plt.ylabel("PC2"); plt.title("PCA on 10 descriptors (n=20)")
# legend
for g, col in group_to_color.items():
plt.scatter([], [], c=col, label=g)
plt.legend(title="Group", loc="best")
plt.tight_layout(); plt.show()
Q3#
# Q3 - Solution
from sklearn.manifold import TSNE
tsne_desc = TSNE(n_components=2, perplexity=2, learning_rate="auto",
init="pca", random_state=0)
Y_desc = tsne_desc.fit_transform(X_desc_std)
plt.figure(figsize=(7,6))
plt.scatter(Y_desc[:,0], Y_desc[:,1], s=80, c=colors, alpha=0.9)
for i, name in enumerate(df10_20["Compound Name"]):
plt.text(Y_desc[i,0]+0.5, Y_desc[i,1], name, fontsize=8)
plt.xlabel("tSNE-1"); plt.ylabel("tSNE-2"); plt.title("t-SNE on 10 descriptors (n=20)")
for g, col in group_to_color.items():
plt.scatter([], [], c=col, label=g)
plt.legend(title="Group", loc="best")
plt.tight_layout(); plt.show()
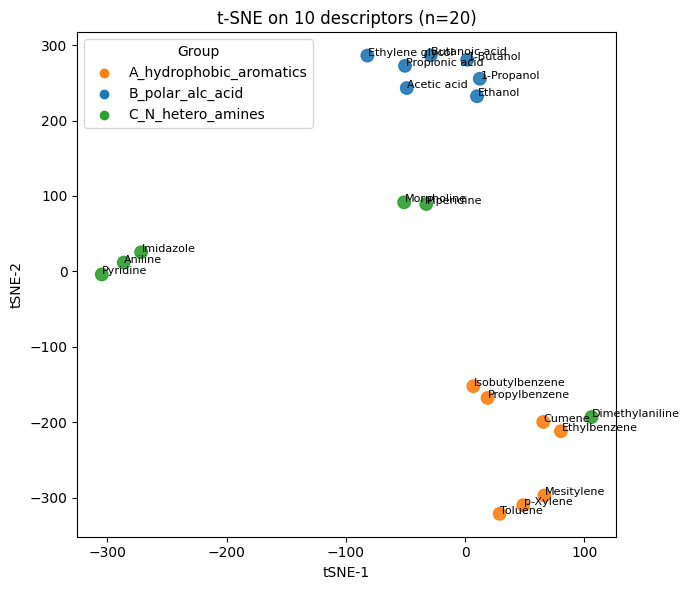
Q4#
# Q4 - Solution
# 0/1 matrix from bitstrings
X_fp = np.array([[int(ch) for ch in s] for s in df_fp_20["Fingerprint"]], dtype=float)
# PCA on fingerprint bits
pca_fp = PCA(n_components=2, random_state=0).fit(X_fp)
Z_fp_pca = pca_fp.transform(X_fp)
plt.figure(figsize=(7,6))
plt.scatter(Z_fp_pca[:,0], Z_fp_pca[:,1], s=80, c=colors, alpha=0.9)
for i, name in enumerate(df_fp_20["Compound Name"]):
plt.text(Z_fp_pca[i,0]+0.03, Z_fp_pca[i,1], name, fontsize=8)
plt.xlabel("PC1"); plt.ylabel("PC2"); plt.title("PCA on Morgan-64 (n=20)")
for g, col in group_to_color.items():
plt.scatter([], [], c=col, label=g)
plt.legend(title="Group", loc="best")
plt.tight_layout(); plt.show()
# t-SNE on fingerprint bits
tsne_fp = TSNE(n_components=2, perplexity=5, learning_rate="auto",
init="pca", random_state=0)
Y_fp = tsne_fp.fit_transform(X_fp)
plt.figure(figsize=(7,6))
plt.scatter(Y_fp[:,0], Y_fp[:,1], s=80, c=colors, alpha=0.9)
for i, name in enumerate(df_fp_20["Compound Name"]):
plt.text(Y_fp[i,0]+0.5, Y_fp[i,1], name, fontsize=8)
plt.xlabel("tSNE-1"); plt.ylabel("tSNE-2"); plt.title("t-SNE on Morgan-64 (n=20)")
for g, col in group_to_color.items():
plt.scatter([], [], c=col, label=g)
plt.legend(title="Group", loc="best")
plt.tight_layout(); plt.show()
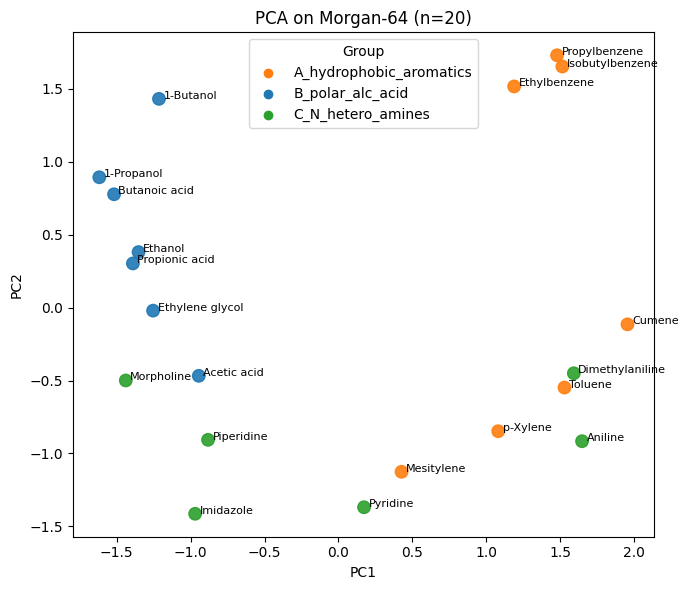
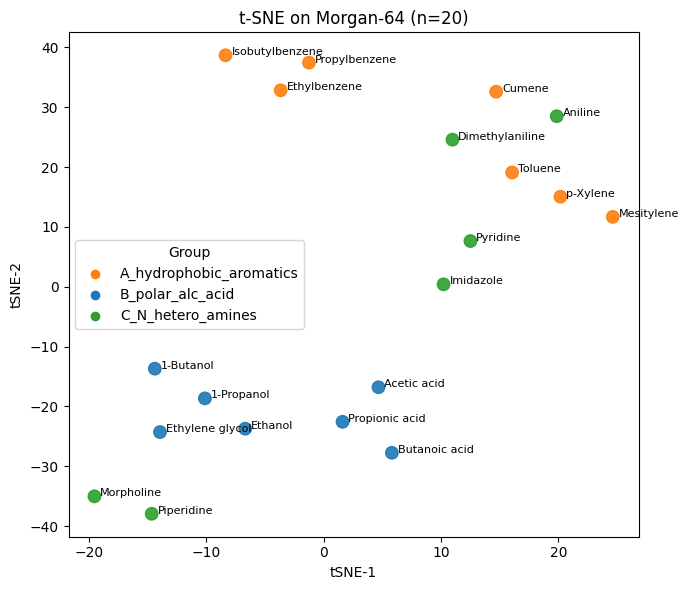
Q5#
# Q5 - Solution
from sklearn.metrics import pairwise_distances
from rdkit import DataStructs
# choose a query row
q = 3 # change during class to test different queries
# 1) Euclidean on standardized 10D descriptors
D_eu = pairwise_distances(X_desc_std, metric="euclidean")
order_eu = np.argsort(D_eu[q])
nn_eu_idx = [i for i in order_eu if i != q][:5]
print(f"Query: {df10_20.loc[q,'Compound Name']} | Group: {df10_20.loc[q,'Group']}")
print("\nNearest 5 by Euclidean (10 descriptors):")
for j in nn_eu_idx:
print(f" {df10_20.loc[j,'Compound Name']:24s} group={df10_20.loc[j,'Group']:22s} d_eu={D_eu[q,j]:.3f}")
# 2) Tanimoto on 64-bit fingerprints
def fp_string_to_bvect(fp_str):
arr = [int(ch) for ch in fp_str]
bv = DataStructs.ExplicitBitVect(len(arr))
for k, b in enumerate(arr):
if b: bv.SetBit(k)
return bv
fps = [fp_string_to_bvect(s) for s in df_fp_20["Fingerprint"]]
S_tan = np.zeros((len(fps), len(fps)))
for i in range(len(fps)):
S_tan[i, :] = DataStructs.BulkTanimotoSimilarity(fps[i], fps)
D_tan = 1.0 - S_tan
order_tan = np.argsort(D_tan[q])
nn_tan_idx = [i for i in order_tan if i != q][:5]
print("\nNearest 5 by Tanimoto (Morgan-64):")
for j in nn_tan_idx:
print(f" {df10_20.loc[j,'Compound Name']:24s} group={df10_20.loc[j,'Group']:22s} d_tan={D_tan[q,j]:.3f}")
Query: p-Xylene | Group: A_hydrophobic_aromatics
Nearest 5 by Euclidean (10 descriptors):
Mesitylene group=A_hydrophobic_aromatics d_eu=0.840
Toluene group=A_hydrophobic_aromatics d_eu=0.859
Ethylbenzene group=A_hydrophobic_aromatics d_eu=1.281
Cumene group=A_hydrophobic_aromatics d_eu=1.572
Dimethylaniline group=C_N_hetero_amines d_eu=2.267
Nearest 5 by Tanimoto (Morgan-64):
Toluene group=A_hydrophobic_aromatics d_tan=0.300
Mesitylene group=A_hydrophobic_aromatics d_tan=0.333
Dimethylaniline group=C_N_hetero_amines d_tan=0.667
Cumene group=A_hydrophobic_aromatics d_tan=0.688
Ethylbenzene group=A_hydrophobic_aromatics d_tan=0.688

